Vancocin 500 Mg Powder For Solution For Infusion And Oral Solution
Package leaflet:
Information for the user
Vancocin 500mg and lOOOmg Powder for Solution for Infusion and Oral Solution
Vancomycin
The name of your medicine is Vancocin 500mg & 1000mg Powder for Solution for Infusion and Oral Solution, which will be referred to as Vancocin throughout the rest of this document. Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
■ Keep this leaflet. You may need to read it again.
■ If you have any further questions, ask your doctor or pharmacist.
■ If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Vancocin is and what it is used for
2. What you need to know before you are given Vancocin
3. How you are given Vancocin
4. Possible side effects
5. How to store Vancocin
6. Contents of the pack and other information
What Vancocin is and what it is used for
Vancocin contains the active ingredient vancomycin hydrochloride, which is an antibiotic. Vancocin powder is made into a solution for infusion or oral solution. Vancocin infusion is used to treat:
■ very serious infections that cannot be treated with other, better tolerated, antibiotics such as penicillins and cephalosporins.
■ severe staphylococcal (a type of bacteria) infections in patients who cannot be given or do not respond to penicillins and cephalosporins, or who have staphylococcal infections that are resistant to other antibiotics.
■ endocarditis (infection of the inside lining of the heart) and to prevent endocarditis in patients at risk from dental or surgical procedures.
■ other infections caused by the staphylococcal bacteria such as bone infection (osteomyelitis), lung tissue infection (pneumonia), blood poisoning (septicaemia) and skin and muscle (soft tissue) infections
Vancomycin can be given orally for the treatment of two specific bacterial gut infections, namely, staphylococcal enterocolitis and pseudomembranous colitis (caused by Clostridium difficile)
2 What you need to know before you are given Vancocin
Do not take Vancocin:
■ If you are allergic to vancomycin.
An allergic reaction may include rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
Warnings and precautions
Talk to your doctor or pharmacist before being given Vancocin if you
■ have an inflammatory disorder of the digestive tract (you may be at risk of side effects, especially if you also have a kidney disorder)
■ have a kidney disorder (you will need to have your blood and kidneys tested during treatment)
■ are elderly (you may need your blood and hearing monitored)
■ have a hearing disorder, especially if you are elderly (you may need hearing tests during treatment)
■ are receiving Vancocin for a long time (you may develop another type of infection)
Other medicines and Vancocin
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. This is especially important of the following, as they may interact with your Vancocin:
■ anaesthetics -these may cause redness, flushing, fainting, collapse or even heart attacks. You should, therefore, tell your doctor that you are taking Vancocin if you are going to have an operation.
■ any drug that affects your nerves or kidneys such as amphotericin B (treats fungal infections), aminoglycosides, bacitracin, polymixin B, colistin, viomycin (antibiotics) or cisplatin
(a chemotherapy drug).
■ Potent diuretics (strong medicines which are given to encourage the production of urine) such as furosemide.
It may still be all right for you to be given Vancocin and your doctor will be able to decide what is suitable for you.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before takingthis medicine.
Driving and using machines
Vancocin should not affect your ability to drive or use machines.
3 How you are given Vancocin
Dosage
The dose of Vancocin will depend on your age, the type of infection you have, and other conditions. Infusions should be given over at least 60 minutes and the solution you are given should not contain more than 5mg/ml of Vancomycin.
The usual adult infusion dose is
500mg every six hours or 1000mg every twelve hours. Before it is given to you, this medicine will initially be dissolved in water and then added to an infusion bag containing sodium chloride (salt) or dextrose (a sugar) solution. It will then be given as a slow infusion through a drip into a vein.
The usual infusion dose for children is
10 mg/kg (body weight) given every 6 hours (total daily dose 40mg/kg of body weight).
The elderly, pregnant mothers, premature infants, and patients with a kidney disorder may need a different dose.
Information for the Health Care Professional
Vancocin
1. NAME OF THE MEDICINAL PRODUCT
Vancocin SOOmg powder for solution for infusion and oral solution.
Vancocin lOOOmg powder for solution for infusion and oral solution.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Vancomycin hydrochloride equivalent to SOOrng vancomycin (525,000 ill). When reconstituted with 10ml water for injections, the solution contains vancomycin 50mg/mi.
Vancomycin hydrochloride equivalent to lOOOmg vancomycin (1,050,000 RJ). When reconstituted with 20ml water for injections, the solution contains vancomycin 50mg/mi.
3. PHARMACEUTICAL FORM
Powder for solution for infusion and oral solution.
An off-white lyophilised plug, when reconstituted in water, it forms a clear solution.
4 CLINICAL PARTICULARS 4.1 Therapeutic indications
Vancomycin is indicated in potentially life threatening infections which cannot be treated with other effective, less toxic antimicrobial drugs, including the penicillins and cephalosporins.
Vancomycin is useful in the therapy of severe staphylococcal infections in patients who cannot receive or who have failed to respond to the penicillins and cephalosporins, or who have infections with staphylococci resistant to other antibiotics.
Vancomycin is used in the treatment of endocarditis and as prophylaxis against endocarditis in patients at risk from dental or surgical procedures.
Its effectiveness has been documented in other infections due to staphylococci, including osteomyelitis, pneumonia, septicaemia and soft tissue infections.
Vancomycin may be used orally for the treatment of staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile. Parenteral administration of vancomycin is not effective for these indications. Intravenous administration may be used concomitantly if required.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
4.2. Posology and method of administration
For intravenous infusion and oral use only and not for intramuscular administration, infusion related adverse events are related to both concentration and rate of administration of vancomycin. Concentrations of no more than 5rng/rnl are recommended, in selected patients in need of fluid restriction, a concentration up to lOrng/rn! may be used; use of such higher concentrations may increase the risk of infusion related events. Infusions should be given over at least 60 minutes. In adults, if doses exceeding SOOrng are used, a rate of infusion of no more than lOrng/min is
i
|
Vancomycin Dosage Guideline for Neonates | ||
|
aPCA Chronological |
Serum Creatinine Dosage | |
|
(Weeks) Age |
Concentration |
(mg/kg) |
|
(Days) |
(mg/dl)b | |
|
<30 £7 |
------C |
15 every 24 hr |
|
>7 |
^1.2 |
10 every 12 hr |
|
30-36 £14 |
10 every 12 hr | |
|
>14 |
£0.6 |
10 every 8 hr |
|
0.7 -1.2 |
10 every 12 hr | |
|
>36 £7 |
10 every 12 hr | |
|
>7 |
£0.6 |
10 every 8 hr |
|
0.7 -1.2 |
10 every 12 hr | |
recommended. Infusion related events may occur, however, at any rate or concentration.
Intravenous Infusion in Patients with Normal Renal Function
Adults: The usual intravenous dose is 500mg every six hours or 1g every 12 hours, in Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous Infusion BP. Each dose should be administered at no more than fOmg/min. Other patient factors, such as age, obesity or pregnancy, may call for modification of the usual daily dose. The majority of patients with infections caused by organisms sensitive to the antibiotic show a therapeutic response within 48-72 hours. The total duration of therapy is determined by the type and severity of the infection and the clinical response of the patient. In staphylococcal endocarditis, treatment for three weeks or longer is recommended.
Pregnancy: It has been reported that significantly increased doses may be required to achieve therapeutic serum concentrations in pregnant patients (see section 4.6 'Fertility, pregnancy and lactation')
The elderly: Dosage reduction may be necessary to a greater extent than expected because of decreasing renal function (see below). Monitor auditory function (see section 4.4 'Special warning and precautions for use'). Paediatric population
The usual intravenous dosage islOmg/kg per dose given every 6 hours (total daily dosage 40rng/kg of body weight). Each dose should be administered over a period of at least 60 minutes.
Neonates have a larger volume of distribution and incompletely developed renal function; therefore dosing guidelines differ from those recommended for children and adults. In neonates and young infants, the total daily dosage may be lower. An initial dose of 15mg/kg is suggested, followed bylOmg/kg every 12 hours in the first week of life and every 8 hours thereafter until one month of age. Each dose should be administered over 60 minutes. Close monitoring of serum vancomycin concentrations may be warranted in these patients. One dosing nomogram for dosing vancomycin in neonates is illustrated in the following table. 1
Patients with impaired renal function:
Dosage adjustments must be made to avoid toxic serum levels. In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function.
Regular monitoring of serum levels is advised in such patients, as accumulation has been reported, especially after prolonged therapy. Vancomycin serum concentrations may be determined by use of a microbiological assay, radioimmunoassay, fluorescence polarisation immunoassay, fluorescence immunoassay or high pressure liquid chromatography.
The following nomogram, based on creatinine clearance values, is provided:
Dosage nomogram for vancomycin in patients with impaired renal function
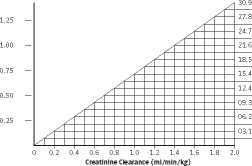
The nomogram is not valid for functionally anephric patients on dialysis. For such patients, a loading dose of 15mg/kg body weight should be given to achieve therapeutic serum levels promptly, and the dose required to maintain stable levels is 1.9rng/kg/24 hours. Since individual maintenance doses of 250rng to 1g are convenient, in patients with marked renal impairment a dose may be given every several days rather than on a daily basis. In anuria a dose of Ig every 7 to 10 days has been recommended.
Measurement of serum concentrations: Following multiple intravenous doses, peak serum concentrations, measured 2 hours after infusion is complete, range from 18-26mg/l. Trough levels measured immediately prior to the next dose should be 5-10mg/l. Ototoxicity has been associated with serum drug levels of 80-100rng/l, but this is rarely seen when serum levels are kept at or below 30mg/l.
Oral Administration
The contents of vials for parenteral administration may be used.
Adults and the elderly: The usual daily dose given is 500rng in divided doses for 7 to 10 days, although up to 2g/day have been used in severe cases. The total daily dosage should not exceed 2g. Each dose may be reconstituted in 30ml water and either given to the patient to drink, or administered by nasogastric tube.
Paediatric population
The usual daily dose is 40mg/kg in three or four divided doses for 7 to 10 days. The total daily dosage should not exceed 2g.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
Capsules are also available.
Preparation of solutions: see section 6.6'Special precautions for disposal and other handling'.
4.3 Contraindications Hypersensitivity to the active substance.
4.4. Special warnings and precautions for use Warnings
Rapid bolus administration (e.g. over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest. Vancomycin should be infused in a dilute solution over a period of not less than 60 minutes to avoid rapid infusion-related reactions. Stopping the infusion usually results in a prompt cessation of these reactions (see 'Posology and method of administration' and 'Undesirable effects' sections).
Some patients with inflammatory disorders of the intestinal mucosa may have significant systemic absorption of oral vancomycin and, therefore, may be at risk for the development of adverse reactions associated with the parenteral administration of vancomycin. The risk is greater in patients with renal impairment. It should be noted that the total systemic and renal clearances of vancomycin are reduced in the elderly.
Due to its potential ototoxicity and nephrotoxicity, vancomycin should be used with care in patients with renal insufficiency and the dose should be reduced according to the degree of renal impairment. The risk of toxicity is appreciably increased by high blood concentrations or prolonged therapy. Blood levels should be monitored and renal function tests should be performed regularly. Vancomycin should also be avoided in patients with previous hearing loss. If it is used in such patients, the dose should be regulated, if possible, by periodic determination of the drug level in the blood. Deafness may be preceded by tinnitus.
The elderly are more susceptible to auditory damage. Experience with other antibiotics suggests that deafness may be progressive despite cessation of treatment. Vancomycin should be administered with caution in patients allergic to teicoplanin, since allergic cross reactions between vancomycin and teicoplanin have been reported. Usage in the elderly: The natural decrement of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted (see section 4.2 'Posology and method of administration'). Paediatric population
In premature neonates and young infants, it may be appropriate to confirm desired vancomycin serum concentrations. Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema and histamine like flushing in children.
Precautions
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin. Therefore, monitoring of serum concentrations may be appropriate in these patients. Patients with borderline renal function and individuals over the age of 60 should be given serial tests of auditory function and of vancomycin blood levels. All patients receiving the drug should have periodic haernatological studies, urine analysis and renal function tests.
Vancomycin is very irritating to tissue, and causes injection site necrosis when injected intramuscularly; it must be infused intravenously. Injection site pain and thrombophlebitis occur in many patients receiving vancomycin and are occasionally severe.
The frequency and severity of thrombophlebitis can be minimised by administering the drug slowly as a dilute solution (2.5 to 5.0g/l) and by rotating the sites of infusion. Prolonged use of vancomycin may result in the overgrowth of non susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should betaken. In rare instances, there have been reports of pseudomembranous colitis, due to C. difficile, developing in patients who received intravenous vancomycin.
4.5. Interaction with other medicinal products and other forms of interaction
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema, histamine-like flushing and anaphylactoid reactions.
There have been reports that the frequency of infusion-related events increases with the concomitant administration of anaesthetic agents. Infusion-related events may be mimmised by the administration of vancomycin as a 60-minute infusion prior to anaesthetic induction.
Concurrent or sequential systemic or topical use of other potentially ototoxic or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymixin B, colistin, viornycin, cisplatin, and loop diuretics may increase the toxicity of vancomycin and if they need to be given should be used with caution and appropriate monitoring.
4.6. Fertility, pregnancy and lactation Pregnancy
Teratology studies have been performed at 5 times the human dose in rats and 3 times the human dose in rabbits, and have revealed no evidence of harm to the foetus due to vancomycin. In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin hydrochloride on infants were evaluated when the drug was administered to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin hydrochloride was found in cord blood. No sensorineural hearing loss or nephrotoxicity attributable to vancomycin was noted. One infant, whose mother received vancomycin in the third trimester, experienced conductive hearing loss that was not attributable to vancomycin. Because vancomycin was administered only in the second and third trimesters, it is not known whether it causes foetal harm. Vancomycin should be given in pregnancy only if clearly needed and blood levels should be monitored carefully to minimise the risk of foetal toxicity. It has been reported, however, that pregnant patients may require significantly increased doses of vancomycin to achieve therapeutic serum concentrations.
Breast-feeding
Vancomycin hydrochloride is excreted in human milk. Caution should be exercised when vancomycin is administered to a nursing woman. It is unlikely that a
|
EUCAST Clinical MIC (version 6.0, valid from 2016-01-01) | ||
|
Microorganism |
Breakpoints (mg/L) | |
|
Susceptible |
Resistant | |
|
Staphylococcus spp. (S. aureus) |
s 21 |
> 2 |
|
Coagu lase- negative staphylococcus |
s 41 |
> 4 |
|
Enterococcus spp. |
s 4 |
> 4 |
|
Streptococcus ABCG |
s 21 |
> 2 |
|
Streptococcus pneumoniae |
£ 21 |
> 2 |
|
Viridans group streptococci |
£ 21 |
> 2 |
|
Gram-positive anaerobes |
£ 2 |
> 2 |
|
Clostridium difficile |
<;72 |
> 72 |
|
Corynebacterium spp. |
£ 2 |
> 2 |
nursing infant can absorb a significant amount of vancomycin from its gastro-intestinal tract.
4.7. Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
Infusion-related events: During or soon after rapid infusion of vancomycin, patients may develop anaphylactoid reactions including hypotension, wheezing, dyspnoea, urticaria or pruritus. Rapid infusion may also cause flushing of the upper-body ('red-neck'syndrorne) or pain and muscle spasm of the chest and back. These reactions usually resolve within 20 minutes but may persist for several hours. In animal studies, hypotension and bradycardia occurred in animals given large doses of vancomycin at high concentrations and rates. Such events are infrequent if vancomycin is given by slow infusion over 60 minutes. In studies of normal volunteers, infusion-related events did not occur when vancomycin was administered at a rate of fOmg/min or less.
Rapid bolus injection may give hypotension, bradycardia, cardiogenic shock and rarely cardiac arrest.
Nephrotoxicity: Rarely, renal failure, principaiiy manifested by increased serum creatinine or blood urea concentrations, have been observed, especially in patients given large doses of intravenously administered vancomycin. Rare cases of interstitial nephritis have been reported. Most occurred in patients who were given aminoglycosides concomitantly or who had pre-existing kidney dysfunction. When vancomycin was discontinued, azotaernia resolved in most patients.
Ototoxicity: Hearing loss associated with intravenously administered vancomycin has been reported. Most of these patients had kidney dysfunction, pre-existing hearing loss, or concomitant treatment with an ototoxic drug. Vertigo, dizziness and tinnitus have been reported rarely. Tinnitus, possibly preceding onset of deafness, may occur and should be regarded as an indication to discontinue treatment.
Haematoiogicai: Reversible neutropenia, usually starting one week or more after onset of intravenous therapy or after a total dose of more than 25g. Neutropenia appears to be promptly reversible when vancomycin is discontinued. Thrombocytopenia has rarely been reported. Reversible agranulocytosis (less than 500 granulocytes per mm1) has been reported rarely, although causality has not been established. Eosinophiiia has been reported.
Miscellaneous: Phlebitis, hypersensitivity reactions anaphylaxis, nausea, chills, drug fever, rashes (including exfoliative dermatitis) and rare cases of vasculitis. Vancomycin has been associated with the bullous eruption disorders, Stevens-Johnson syndrome, toxic epidermal necrolysis and linear IgA bullous dermatosis. If a bullous disorder is suspected, the drug should be discontinued and specialist dermatological assessment should be carried out. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continues monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme Website: www.mhra.gov.uk/yeilowcard.
4.9. Overdose
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed from the blood by haemodialysis or peritoneal dialysis. Haernoperfusion with Arnberlite resin XAD-4 has been reported to be of limited benefit.
5 PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties
Pharmacotherapeutic group: glycopeptide antibacterials ATC Code: J01 XA01 for intravenous use and A07 AA09 for oral use.
Vancomycin is a tricyclic glycopeptide antibiotic derived from Amycotatopsis orientalis. The primary mode of action of vancomycin is inhibition of cell-wall synthesis, in addition, vancomycin may alter bacterial ceil membrane permeability and RNA synthesis. There is no cross-resistance between vancomycin and other classes of antibiotics.
EUCAST Clinical MIC Breakpoints
1 Non-susceptible isolates are rare or not yet reported. The identification and antimicrobial susceptibility test result on any such isolate must be confirmed and the isolate sent to a reference laboratory.
2 The breakpoints are based on epidemiological cut off values (ECOFFs), which distinguish wild type isolates from those with reduced susceptibility.
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Commonly susceptible species:
Gram-positive aerobes Enterococcus faecatis Staphylococcus aureus Coagu lase- negative sta phyioccoci Streptococcus group B Streptococcus group C Streptococcus group G Streptococcus pneumoniae
Streptococcus pyogenes Viridans streptococci
Species for which acquired resistance may be a problem: Gram-positive aerobes
Enterococcus faecium
Clostridium difficile (e.g. toxigenic strains implicated in pseudomembranous colitis) is a target species for oral use where high intraluminal concentrations of vancomycin are achieved.
5.2. Pharmacokinetic properties
Vancomycin is given intravenously for therapy of systemic infections.
In subjects with normal renal function, multiple intravenous dosing of fg of vancomycin (15mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63rng/L immediately after the completion of infusion, mean plasma concentrations of approximately 23mg/L 2 hours after infusion. Multiple dosing of SOOmg infused over 30 minutes produces mean plasma concentrations of about 49rng/L at the completion of infusion, mean plasma concentrations of about 19mg/L 2 hours after infusion, and mean plasma concentrations of about lOrng/L 6 hours after infusion. The plasma concentrations during multiple dosing are similar to those after a single dose.
The mean elimination half-life of vancomycin from the plasma is 4 to 6 hours in patients with normal renal function. About 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration in the first 24 hours.
Mean plasma clearance is about 0.058 L/kg/h, and mean renal clearance is about 0.048L/kg/h. Renal vancomycin clearance is fairly constant and accounts for 70% to 80% of vancomycin elimination. The volume of distribution ranges from 0.39 to 0.97L/kg. There is no apparent metabolism of the drug. Vancomycin is 55% protein bound as measured by ultrafiltration at vancomycin serum levels of 10 to 100mg/L. After IV administration of vancomycin hydrochloride, inhibitory concentrations are present in pleural, pericardial, ascitic, atrial appendage tissue and synovial fluid, as well as urine and peritoneal fluid. Vancomycin does not readily penetrate the cerebrospinal fluid unless the meninges are inflamed.
Renal dysfunction slows excretion of vancomycin, in anephric patients, the average half-life of elimination is 7.5 days.
The total systemic and renal clearance of vancomycin may be reduced in the elderly due to the natural decrement of glomerular filtration.
Vancomycin is not significantly absorbed from the normal gastro intestinal tract and is therefore not effective by the oral route for infections other than staphylococcal enterocolitis and pseudomembranous colitis due to
Clostridium difficile.
Orally administered vancomycin does not usually enter the systemic circulation even when inflammatory lesions are present. Measurable serum concentrations may occur infrequently in patients with active C. difficile-induced pseudomembranous colitis and, in the presence of renal impairment, the possibility of accumulation exists. Administration of vancomycin oral solution, 2g daily for 16 days to anephric patients with no inflammatory bowel disease, gave serum levels of <0.66|jg/ml. With doses of 2g daily, concentration of 3,100mg/kg can be found in the faeces and levels of <1 pg/ml can be found in the serum of patients with normal renal function who have pseudomembranous colitis.
5.3. Pre-clinical Safety Data Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no mutagenic potential of vancomycin was found in standard laboratory tests. No definitive fertility studies have been performed.
6 PHARMACEUTICAL PARTICULARS 6.1 List of excipients
None
(Vials of Vancocin contain only the active ingredient, vancomycin hydrochloride).
6.2. Incompatibilities
Vancomycin solution has a low pH that may cause chemical or physical instability when it is mixed with other compounds. Mixing with alkaline solutions should be avoided.
Mixtures of solutions of vancomycin and beta-lactam antibiotics have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush intravenous lines between administration of these antibiotics. It is also recommended to dilute solutions of vancomycin to 5rng/rnL or less.
Although intravitreal injection is not an approved route of administration for vancomycin, precipitation has been reported after intravitreal injection of vancomycin and ceftazidime for endophthalmitis using different syringes and needles. The precipitates dissolved gradually, with complete clearing of the vitreous cavity over two months and with improvement of visual acuity.
6.3. Shelf life 2 yea rs
After reconstitution: May be stored in a refrigerator (2°-8°C) for 24 hours.
Prior to administration, parenteral drug products should be inspected visually for particulate matter and discolouration whenever solution or container permits.
Solutions of the parenteral powder intended for oral administration may be stored in a refrigerator (2°-8°C) for 96 hours.
6.4. Special precautions for storage
Do not store above 25°C.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
500mg presentation: Rubber stoppered 10m! vials each containing chromatographically purified vancomycin hydrochloride 525,OOOiu, equivalent to 500mg vancomycin as an off-white iyophilised plug One 10m! vial packaged in a cardboard carton, lg presentation: Rubber stoppered 20ml vials containing chromatographically purified vancomycin hydrochloride, 1,050,000111, equivalent to 1 g vancomycin, as an off-white Iyophilised plug.
One 20ml vial packaged in a cardboard carton.
6.6 Special precautions for disposal and other handling
Preparation of solution: At the time of use, add 10ml of Water for Injections PhEur to the 500mg vial, or 20ml Water for Injections PhEur to the lOOOrng vial. Vials reconstituted in this manner will give a solution of 50rng/mi.
FURTHER DILUTION IS REQUIRED. Read instructions which follow:
Intermittent infusion is the preferred method of administration. Reconstituted solutions containing 500mg vancomycin must be diluted with at least 100m! diluent. Reconstituted solutions containing lOOOrng vancomycin must be diluted with at least 200ml diluent. Sodium Chloride Intravenous infusion BP or 5% Dextrose Intravenous Infusion BP are suitable diluents. The desired dose should be given by intravenous infusion over a period of at least 60 minutes. If administered over a shorter period of time or in higher concentrations, there is the possibility of inducing marked hypotension in addition to thrombophlebitis. Rapid administration may also produce flushing and a transient rash over the neck and shoulders. Continuous infusion (should be used only when intermittent infusion is not feasible). 1000-2000rng can be added to a sufficiently large volume of Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous infusion BP to permit the desired daily dose to be administered slowly by intravenous drip over a 24 hour period.
Oral Administration
The contents of vials for parenteral administration may be used.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
Capsules are also available.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Flynn Pharma Ltd Alton House 4 Herbert Street Dublin 2 Ireland
8. MARKETING AUTHORISATION NUMBER(S)
PL 13621 /0033
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 18/04/1990 Date of latest renewal: 11/10/2005
10 DATE OF REVISION OF THE TEXT
04/05/2016
FLYNN
PHARMA
LTD
The usual adult oral dose is 500mg per day in divided doses for 7 to 10 days. The maximum daily dose is 2g/day for severe infections. Each dose can be added to 30ml water and either given to the patient to drink, or given by nasogastric tube (a tube passing through the nose into the stomach).
The usual oral dose for children is
40mg/kg (body weight) in three or four doses for 7 to 10 days. The maximum daily dose is 2g.
If you are given more Vancocin than you should receive
As Vancocin will be given to you whilst you are in hospital is unlikely that you will be given too little or too much, however, tell your doctor or nurse if you have any concerns.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. All medicines can cause allergic reactions, although serious allergic reactions are very rare. Tell your doctor immediately if you get any sudden wheeziness, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching (especially affecting your whole body).
Serious side effects
The following side effects are serious. You should stop takingthis medicine and tell your doctor immediately if you experience them:
■ serious peeling or blistering of the skin
■ severe allergic reactions including shortness of breath, wheezing, fainting, collapse, very slow pulse rate, swollen hands and face, and hearing loss
The following side effects have been reported:
■ changes in blood counts, which will show upas bruising or a very tired feeling (a blood test carried out by a doctor will detect these)
■ chills
■ fever
■ rash (alone)
■ itching
■ inflammation of the vein where your drip is inserted
■ you may stop urinating or notice that you are urinating much less than usual (kidney failure) orthat your urine becomes cloudy
■ spinning sensation (vertigo)
■ dizziness
■ ringing in the ears (tinnitus)
■ hearing loss
■ rarely it can cause cardiac arrest (heart attack).
Other possible side effects:
If Vancocin is infused too quickly, or given as a very concentrated solution, patients may develop severe allergic reactions as described above. Rapid infusion may also cause flushing of the upper body ('red neck'syndrome) or pain and muscle spasms in the chest and back. Your doctor will ensure that Vancocin is infused slowly and is given at the correct concentration
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this ieaflet. You can also report side effects directly via the Yellow Card Scheme Website: www.mhra.gov.uk/ yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
How to store Vancocin
Your doctor or pharmacist knows how to store Vancocin.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of that month.
Before it is made up it should be stored below 25°C.
After it is made up, Vancocin solution for infusion may be stored in a refrigerator (2°-8°C) for 24 hours. Solutions intended for oral dosing may be stored in a refrigerator (2°-8°C) for 96 hours.
Your doctor will ensure that the solution is not discoloured or contains particles.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the envi ronment.
6 Contents of the pack and other information
What Vancocin Powder contains:
The active substance is vancomycin hydrochloride 500mg orlOOOmg.
There are no other ingredients.
What Vancocin looks like and contents of the pack
500mg presentation: Rubber stoppered 10ml vials each containing vancomycin hydrochloride equivalent to 500 mg vancomycin as an off-white powder.
One 10 ml vial packaged in a cardboard carton. lOOOrng presentation: Rubber stoppered 20ml vials containing vancomycin hydrochloride, equivalent to 1000 mg vancomycin, as an off-white powder.
One 20ml vial packaged in a cardboard carton.
Marketing Authorisation Holder
Flynn Pharma Ltd Alton House 4 Herbert Street Dublin 2,
Ireland
Manufacturer
Xellia Pharmaceuticals ApS Dalslandsgade 11 DK-2300 Kpbenhavn S Denmark
This leaflet was last revised In May 2016
FLYNN
PHARMA
LTD
PCA = postconceptual age (gestational age at birth plus chronological age)
b If the serum creatinine concentration is >1.2mg/dl, use an initial dosage of 15mg/kg every 24 hours c Serum creatinine concentration is not used to determine the dosage for this type of patient because of its lack of reliability or because of the lack of information.