Vancomycin 1000 Mg Powder For Concentrate For Solution For Infusion
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Vancomycin 1000 mg Powder for concentrate for solution for infusion.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 1000 mg vancomycin (as vancomycin hydrochloride) equivalent to 1,000,000 IU.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for concentrate for solution for infusion ‘A white to cream coloured porous cake’
After reconstitution a solution is obtained with a pH of approximately 3.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Vancomycin is indicated in adults, infants, children aged one month to 12 years and adolescents over 12 years.
Intravenous vancomycin is indicated in the following severe infections caused by gram-positive bacteria susceptible to vancomycin which cannot be treated or failed to respond or are resistant to other antibiotics such as penicillins and cephalosporins (see section 5.1).
- endocarditis
- infections of the bones (osteomyelitis)
- pneumonia
soft tissue infections
Where appropriate, vancomycin should be co-administered with other antibacterial agents. This particularly applies to the treatment of endocarditis. Vancomycin may be used for the perioperative prophylaxis against bacterial endocarditis, in patients at high risk of developing bacterial endocarditis when they undergo major surgical procedures (e.g., cardiac and vascular procedures, etc) and are unable to receive a suitable beta-lactam antibacterial agent.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
4.2 Posology and method of administration
Posology
Patients requiring fluid restriction can receive a solution of 500 mg / 50 ml or 1000 mg / 100 ml. With these higher concentrations the risk for infusion related side effects can be increased. Infusion related events may occur, however, at any rate or concentration.
Intravenous use (infusion) in patients with normal renal function:
Adults and adolescents above 12 years of age:
The recommended daily intravenous dose is 2000 mg; divided into doses of 500 mg every 6 hours or 1000 mg every 12 hours
For bacterial endocarditis, the generally accepted regimen is 1000 mg (1g) vancomycin intravenously every 12 hours for 4 weeks either alone or in combination with other antibiotics. Longer treatment may be required, depending on the pathogen involved. National guidance should be followed.
Peri-operative prophylaxis against bacterial endocarditis:
It is commonly recommended that adults receive 1000 mg (1g) vancomycin intravenously prior to surgery (prior to induction of anaesthesia) and depending on duration and type of surgery, another dose of 1000 mg (1g) of vancomycin i.v. may be given after 12 hours. National guidance should be followed.
Children one month to 12 years of age:
The usual intravenous dosage is 10mg/kg per dose given every six hours (total daily dosage 40mg/kg of body weight). Each dose should be administered over a period of at least 60 minutes.
Newborn infants (full-term):
0-7 days of age: A starting dose of 15 mg/kg, followed by 10 mg/kg every 12 hours.
7-30 days of age: A starting dose of 15 mg/kg, followed by 10 mg/kg every 8 hours.
Each dose should be administered over 60 minutes. Close monitoring of serum vancomycin concentrations may be warranted in these patients.
Pregnancy:
It has been reported that significantly increased doses may be required to achieve therapeutic serum concentrations in pregnant patients - see Section 4.6 Fertility, pregnancy and lactation
The elderly:
Dosage reduction may be necessary because of decreasing renal function (see below).
Obese patients:
Modification of the usual daily doses may be required.
Patients with hepatic insufficiency:
There is no evidence that the dose has to be reduced in patients with hepatic insufficiency.
Patients with impaired renal function:
Dosage adjustments must be made to avoid toxic serum levels, hence serum levels of vancomycin should be monitored regularly.
The following nomogram, based on creatinine clearance values, is provided as a guidance for dose adjustments:
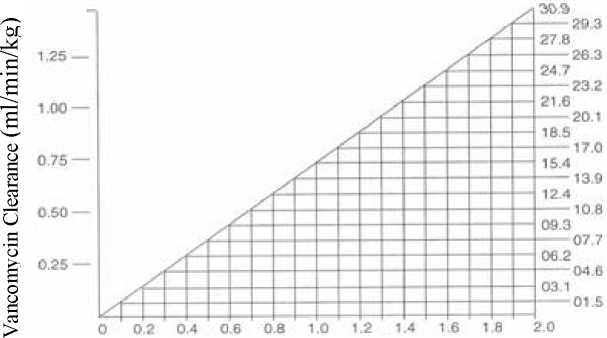
Creatinine Clearance (ml/min/kg)t
Dosage nomogram for vancomycin in patients with impaired renal function
The nomogram is not valid for functionally anephric patients on dialysis. For such patients, a loading dose of 15mg/kg body weight should be given to achieve therapeutic serum levels promptly, and the dose required to maintain stable levels is 1.9mg/kg/24 hours. Since individual maintenance doses of 250mg to 1g are convenient, in patients with marked renal impairment a dose may be given every several days rather than on a daily basis. In anuria a dose of 1g every seven to ten days has been recommended.
If polysulfone membranes are used for hemodialysis („high flux dialysis“), the half time of vancomycin is shortened. For patients with regular hemodialysis an additional maintenance dose may be necessary.
If serum creatinine level alone is available, the following formula may be applied to calculate the creatinine clearance:
Men: Weight (kg) x (140 - age (years))
72 x serum creatinine (mg/100ml)
Women: 0.85 x value calculated by the above formula
Monitoring of vancomycin serum concentrations:
The serum concentration of vancomycin should be monitored at the second day of treatment immediately prior to the next dose, and one hour post infusion. Therapeutic vancomycin blood levels should be between 30 and 40 mg/l (maximum 50 mg/l) one hour after the end of the infusion, the minimum level (short prior to the next administration) between 5 and 10 mg/l.
The concentrations should normally be monitored twice or three times per week.
Duration of treatment
The duration of the treatment depends on the severity of the infection as well as on the clinical and bacteriological progress.
Method of administration:
Parenterally vancomycin shall only be administered as slow intravenous infusion (not more than 10 mg/min - over at least 60 min) which is sufficiently diluted (at least 100 ml per 500 mg or at least 200 ml per 1000 mg).
Patients requiring fluid restriction can receive a solution of 500 mg / 50 ml or 1000 mg / 100 ml. With these higher concentrations the risk for infusion related side effects can be increased. Infusion related events may occur, however, at any rate or concentration.
For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Rapid bolus administration (eg, over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest, histamine like responses and maculopapular or erythematous rash (“red man’s syndrome” or “red neck syndrome”). Vancomycin should be infused in a dilute solution over a period of not less than 60 minutes to avoid rapid infusion-related reactions. Stopping the infusion usually results in a prompt cessation of these reactions (see section 4.2 and section 4.8).
In case of severe acute hypersensitivity reactions (e.g. anaphylaxis), the treatment with vancomycin has to be discontinued immediately and the usual appropriate emergency measures have to be started.
Vancomycin should be used with caution in patients with allergic reactions to teicoplanin, since cross hypersensitivity reactions between vancomycin and teicoplanin have been reported.
Due to its potential ototoxicity and nephrotoxicity, vancomycin should be used with care in patients with renal insufficiency and the dose should be reduced according to the degree of renal impairment. The risk of toxicity is appreciably increased by high blood concentrations or prolonged therapy. Blood levels should be monitored and renal function tests should be performed regularly. Ototoxicity, which may be transitory or permanent (see section 4.8) has been reported in patients with prior deafness, who have received excessive intravenous doses, or who receive concomitant treatment with another ototoxic active substance such as aminoglycosides. Deafness may be preceded by tinnitus. Experience with other antibiotics suggests that deafness may be progressive despite cessation of treatment. The elderly are more susceptible to auditory damage. Vancomycin should be avoided in patients with previous hearing loss. If it is used in such patients, blood levels should be determined periodically and periodic testing of auditory function is recommended.
Use in paediatrics: In premature neonates and young infants, it may be appropriate to confirm desired vancomycin serum concentrations.
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema and histamine like flushing in children.
Use in the elderly: The natural decrement of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted (see 'Posology and method of administration').
Precautions
Regular monitoring of the blood levels of vancomycin is indicated in longer-term use, particularly in patients with renal dysfunction or impaired faculty of hearing as well as in concurrent administration of nephrotoxic or ototoxic substances, respectively.
Doses should be titrated on the basis of serum levels. Blood levels should be monitored and renal function tests performed regularly. It is a general recommendation to monitor plasma concentrations 2-3 times weekly.
Patients with borderline renal function and individuals over the age of 60 should be given serial tests of auditory function and of vancomycin blood
levels. All patients receiving the drug should have periodic haematological studies, urine analysis and renal function tests.
Vancomycin is very irritating to tissue, and causes injection site necrosis when injected intramuscularly; it must be infused intravenously. Injection site pain and thrombophlebitis occur in many patients receiving vancomycin and are occasionally severe.
The frequency and severity of thrombophlebitis can be minimised by administering the drug slowly as a dilute solution (2.5 to 5.0g/l) and by rotating the sites of infusion.
Prolonged use of vancomycin may result in the overgrowth of non-susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis, due to C. difficile, developing in patients who received intravenous vancomycin.
As cases of cross hypersensitivity have been reported, Vancomycin must be administred with care in patients with known hypersensitivity to Teicoplanin.
4.5 Interaction with other medicinal products and other forms of interaction
Anaesthetic agents
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema, histamine-like flushing and anaphylactoid reactions. There have been reports that the frequency of infusion-related events increases with the concomitant administration of anaesthetic agents. Infusion-related events may be minimised by the administration of vancomycin as a 60-minute infusion prior to anaesthetic induction.
Potentially nephro- or ototoxic medicinal products
Concurrent or sequential systemic or topical use of other potentially ototoxic, neurotoxic, or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymyxin B, colistin, viomycin or cisplatin, when indicated, requires careful monitoring as the risk for oto- or nephrotoxicity is increased. Muscle relaxants
There is an increased potential of prolonged neuromuscular blockade with concomitant administration of vancomycin and neuromuscular blocking agents.
4.6 Fertility, pregnancy and lactation
Pregnancy:
No sufficient safety experience is available regarding vancomycin during human pregnancy. Reproduction toxicological studies on animals do not suggest any effects on the development of the embryo, foetus or gestation period (see section 5.3).
However, vancomycin penetrates the placenta and a potential risk of embryonal and neonatal ototoxicity and nephrotoxicity cannot be excluded.
Therefore vancomycin should be given in pregnancy only if clearly needed and after a careful risk/benefit evaluation.
Lactation Vancomycin is excreted in human milk and should be therefore used in lactation period only if clearly necessary. Vancomycin should be cautiously given to breast-feeding mothers because of potential adverse reactions in the infant (disturbances in the intestinal flora with diarrhoea, colonisation with yeast-like fungi and possibly sensibilisation).
Considering the importance of this medicine for nursing mother, the decision to stop breastfeeding should be considered.
4.7 Effects on ability to drive and use machines
Vancomycin has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
The adverse reactions listed below are defined using the following MedDRA: Very common (>1/10); Common (>1/100—to <1/10); Uncommon (>1/1,000, to <1/100); Rare (>1/10,000 - to <1/1,000); Very rare (<1/10,000); not known (cannot be estimated from the available data)
Intravenous infusion:
The most common adverse reactions are phlebitis and pseudo-allergic reactions in connection with too rapid intravenous infusion of vancomycin (see section 4.4).
Blood and the lymphatic system disorder:
Rare (>1/10,000 to <1/1,000): thrombocytopenia, neutropenia, agranulocytoses, eosinophilia.
Immune system disorders
Rare (> 1/10,000 to <1/1,000): anaphylactic reactions, hypersensitivity reactions
Ear and labyrinth disorders:
Uncommon (>1/1,000 to <1/100): Transient or permanent loss of hearing.
Rare (>1/10,000 to <1/1,000): Tinnitus, dizziness.
Cardiac disorders:
Very rare (<1/10,000): cardiac arrest
Vascular disorders:
Common (>1/100 to <1/10): Decrease in blood pressure, thrombophlebitis. Rare (>1/10,000 to <1/1,000): Vasculitis
Respiratory, thoracic and mediastinal disorders:
Common (>1/100 to <1/10): Dyspnoea, stridor.
Gastrointestinal disorders:
Rare (>1/10,000 to <1/1,000): Nausea, diarrhoea.
Very rare (<1/10,000): Pseudomembranous enterocolitis.
Skin and subcutaneous tissue disorders:
Common (>1/100 to <1/10): Exanthema and mucosal inflammation, pruritus, urticaria.
Very rare (<1/10,000): Exfoliative dermatitis, Stevens-Johnson syndrome, Lyell's syndrome, Linear IgA bullous dermatosis.
Renal and urinary disorders:
Common (>1/100 to <1/10): Renal insufficiency manifested primarily by increased serum creatinine.
Rare (>1/10,000 to <1/1,000): Interstitial nephritis, acute renal failure.
General disorders and administration site conditions:
Common (>1/100 to <1/10): Phlebitis, redness of the upper body and the face. Rare (>1/10,000 to <1/1,000): Drug fever, shivering. Pain in the chest and back muscles.
Infusion related events:
During or shortly after rapid infusion anaphylactoid reactions may occur, including hypotension, dyspnea, urticaria or pruritus. Redness of the skin on the upper body (Red man syndrome), pain and cramps in chest or back muscle can occur.
The reactions abate when administration is stopped, generally between 20 minutes and 2 hours. Vancomycin should be infused slowly (for more than 60 minutes - see section 4.4).
Ototoxicity may be reversible or permanent, and has been reported mainly in patients given an overdose in patients with a history of reduced hearing, and with concomitant therapy with other ototoxic drugs, such as aminoglycosides.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow card scheme; tel: Freephone 0808 100 3352, website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed from the blood by haemodialysis or peritoneal dialysis. Haemoperfusion with Amberlite resin XAD-4 has been reported to be of limited benefit.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group, “antibacterials for systemic use Glycopeptide Antibacterials’”. “ATC Code: J01X A01”.
Mechanism of action:
Vancomycin is a tricyclic glycopeptide antibiotic that inhibits the syntesis of the cell wall in sensitive bacteria by binding with high affinity to the D-alanyl-D-alanine terminus of cell wall precursor units. The drug is bactericidal for dividing microorganisms.
Pharmacodynamic effects, PK/PD relationship:
Vancomycin displays concentration-independent activity with the area under the concentration curve (AUC) divided by the minimum inhibitory concentration (MIC) of the target organism as the primary predictive parameter for efficacy.
Mechanism of resistance:
Acquired resistance to glycopeptides is most common in enterococci and is based on acquisition of various van gene complexes which modifies the D-alanyl-D-alanine target to D-alanyl-D-lactate or D-alanyl-D-serine which bind vancomycin poorly. Cross-resistance with teicoplanin has been reported for some van genes. The reduced susceptibility or resistance to vancomycin in Staphylococcus is not well understood. Van genes have rarely been found in Staphylococcus aureus, where changes in cell wall structure result in “intermediate” susceptibility, which is most commonly heterogeneous. Susceptibility:
Vancomycin is active against gram-positive bacteria . Gram-negative bacteria are resistant.
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Breakpoints
EUCAST (European Committee on Antimicrobial Susceptibility testing) recommendations
|
Susceptible |
Resistant | |
|
Staphylococcus spp. |
< 2 mg/L |
> 2 mg/L |
|
Enterococcus spp. |
< 4 mg/L |
> 4 mg/L |
|
Streptococcus spp |
< 2 mg/L |
> 2 mg/L |
|
Streptococcus pneumoniae |
< 2 mg/L |
> 2 mg/L |
|
Grampositive anaerobes |
< 2 mg/L |
< 2 mg/L |
|
Non species related* |
< 2 mg/L |
> 4 mg/L |
*Non-species related breakpoints have been determined mainly on the basis of PK/PD data and are independent of MIC distributions of specific species. They are for use only for species that have not been given a species-specific breakpoint and not for those species where susceptibility testing is not recommended.
Classes_
Commonly susceptible species_
Gram positive
Enterococcus faecalis Staphylococcus aureus Staphylococcus coagulase negative Streptococcus spp.
Streptococcus pneumoniae
Clostridium spp._
Species for which acquired resistance may be a problem_
Enterococcus faecium_
Inherently resistant_
Gram-negative bacteria Chlamydia spp.
Mycobacteria Mycoplasma spp.
Rickettsia spp._
5.2 Pharmacokinetic properties
Absorption
Vancomycin is administered intravenously for the treatment of systemic infections. In the case of patients with normal renal function, intravenous infusion of multiple doses of 1g vancomycin (15 mg/kg) for 60 minutes produces approximate average plasma concentrations of 50-60 mcg/mL, 20-25 mcg/mL and 5-10 mcg/mL, immediately, 2 hours and 11 hours after completing the infusion, respectively. Intravenous infusion of multiple doses of 500 mg for 30 minutes produces average plasma concentrations of around 40-50 mg/l, 19-20 mg/l and 10-11 mg/l immediately, 2 hours and 6 hours after completing the infusion, respectively. The plasma levels obtained after multiple doses are similar to those achieved after a single dose.
In case of oral use, high-polar vancomycin is virtually not absorbed. It appears after oral administration in active form in the stool, and is therefore a suitable chemotherapeutic for pseudomembranous colitis and staphylococcal colitis.
Distribution
At serum concentrations of vancomycin of 10 mg/l to 100 mg/l, the binding of the drug to plasma proteins is approximately 30-55%, measured by ultrafiltration.
After intravenous administration of vancomycin hydrochloride, inhibitory concentrations are found in the pleural, pericardial, ascitic and sinovial fluids, in the urine and the peritoneal dialysis fluid and in the tissue of the atrial appendix.
In non-inflamed meninges vancomycin passes the blood-brain barrier only to a low extent.
Elimination
The elimination half-life of vancomycin is 4 to 6 hours in patients with normal renal function and 2,2-3 hours in children. In the first 24 hours, approximately 80 % of an administered dose of vancomycin is excreted in the urine through glomerular filtration. Renal dysfunction delays the excretion of vancomycin.
In anephric patients, the mean half-life is 7.5 days. There is very little metabolism of the drug. Approximately 35-65% of an intraperitoneal dose of vancomycin administered during peritoneal dialysis is absorbed systemically in six hours. Serum concentrations of approximately 8 mg/litre are achieved through intraperitoneal injection of 30 mg/kg vancomycin. Although the vancomycin is not eliminated efficiently by haemodialysis or peritoneal dialysis, there have been reports of an increase in vancomycin clearance with haemoperfusion and haemofiltration. Total systemic and renal clearance of vancomycin may be reduced in persons of advanced age.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity.
Limited data on mutagenic effects show negative results, long-term studies in animals regarding a carcinogenic potential are not available. In teratogenicity studies, where rats and rabbits received doses approximately corresponding to the human dose based on body surface (mg/m2), no direct or indirect teratogenic effects were observed.
Animal studies of the use during the perinatal/postnatal period and regarding effects on fertility are not available.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
None
6.2 Incompatibilities
Vancomycin solution has a low pH that may cause chemical or physical instability when it is mixed with other compounds. Mixing with alkaline solutions should be avoided. Each parenteral solution should be checked visually for precipitation and discolouration prior to use.
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life
Powder as packaged for sale:
2 years
Reconstituted concentrate:
The reconstituted concentrate should be further diluted immediately after reconstitution.
Diluted product:
From a microbiological point of view, the product should be used immediately.
6.4 Special precautions for storage
Powder as packaged for sale:
Store below 25°C.
Keep the vial in the outer carton in order to protect from light. Reconstituted concentrate and diluted product:
For storage conditions after reconstitution and further dilution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Colourless type 1, 20 ml glass vial, with a chlorobutyl type 1 silicone coated stopper and a green aluminium/polypropylene flip-off cap.
Pack sizes: 1 vial
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
The product must be reconstituted and the resulting concentrate must then be diluted prior to use.
Preparation of the reconstituted concentrate:
Dissolve the content of each 1000 mg vial in 20 ml of sterile water for injections.
Appearance of reconstituted concentrate:
Clear and colourless solution, free from particles.
One ml of reconstituted concentrate contains 50 mg of vancomycin.
For storage conditions of the reconstituted concentrate, see sections 6.3
Preparation of final diluted solution infusion:
Reconstituted concentrate containing 50 mg/ml of vancomycin should be further diluted immediately after reconstitution.
Suitable diluents are:
Sodium Chloride 9 mg/ml (0.9%) Injection, Glucose 50 mg/ml (5%)
Injection, Sodium Chloride 9 mg/ml (0.9 %) and Glucose 50 mg/ml (5%) Injection or Ringer acetate Injection.
Before administration, the reconstituted and diluted solutions should be inspected visually for particulate matter and discoloration. Only clear, and colourless solution free from particles should be used.
Intermittent infusion:
Reconstituted concentrate containing 1000 mg of vancomycin (50 mg/ml) must be diluted further with at least 200 ml diluent immediately after reconstitution.
The concentration of vancomycin in Solution for infusion should not exceed 5 mg/ml.
The desired dose should be administered slowly by intravenous infusion at a rate of no more than 10 mg/minute, for at least 60 minutes or even longer.
For storage conditions of the diluted medicinal product, see sections 6.3
Disposal
Vials are for single use only. Unused product must be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Actavis Group PTC ehf.
Reykjavikurvegi 76-78 220 HafnarfjorSur Iceland
8 MARKETING AUTHORISATION NUMBER(S)
PL 30306/0658
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
19/01/2015
10 DATE OF REVISION OF THE TEXT
04/03/2015