Vancomycin 1000 Mg Powder For Concentrate For Solution For Infusion
Out of date information, search another

PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
Vancomycin 500 mg
powder for concentrate for solution for infusion Vancomycin 1000 mg
powder for concentrate for solution for infusion
Vancomycin
Read all of this leaflet carefully before
you start taking this medicine because it
contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
The name of your medicine is one of the
following:
- Vancomycin 500 mg Powder for Concentrate for Solution for Infusion
- Vancomycin 1000 mg Powder for Concentrate for Solution for Infusion
1. What is Vancomycin and what it is used for
2. What you need to know before you use Vancomycin
3. How to use Vancomycin
4. Possible side effects
5. How to store Vancomycin
6. Contents of the pack and other information
1. WHAT IS VANCOMYCIN AND WHAT IT IS USED FOR
Vancomycin belongs to a group of glycopeptide antibiotics which eliminate bacteria that cause many kinds of infections, including pneumonia and skin, bone and heart valve infections.
It is used to treat:
- serious infections caused by vancomycin-sensitive bacteria which are resistant (insensitive) to many other antibiotics;
- patients allergic to penicillins and cephalosporins.
It can also be given to you before some surgical procedures to prevent infections.
Your medicine is in the form of a powder for solution. Before use, it will be dissolved and diluted with an intravenous fluid that will be given to you slowly by a drip into your vein by a doctor or nurse.

If you will receive vancomycin over a longer-term period, your blood will be tested at regular intervals. You should also be monitored because of possible superinfection (new infection occurring over the existing one) or severe, sometimes bloody diarrhoea (condition called pseudomembranous colitis).
Other medicines and Vancomycin
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription, herbal remedies or vitamins and minerals, because some of them could have an interaction with vancomycin. Furthermore, do not take any new medicine without consulting your doctor.
The following medicines can react with vancomycin if you take them at the same time, such as medicines for the treatment of:
• infections caused by bacteria
(streptomycin, neomycin, gentamicin, kanamycin, amikacin, bacitracin, tobramycin, polymixin B, colistin),
• tuberculosis (viomycin),
• fungal infections (amphotericin B),
• cancer (cisplatin) and
• medicines for muscle relaxation during anaesthesia,
• anaesthetic agents (if you are going to have general anaesthesia).
Your doctor may need to monitor your blood and adjust the dosage if vancomycin is given at the same time with other medicines.
Pregnancy and breast-feeding
Pregnancy
If you are, or think you may be, pregnant, tell your doctor. Vancomycin should be given during pregnancy only if clearly needed.
Breast-feeding
Tell your doctor if you are breast-feeding as Vancomycin passes into breast milk. Your doctor will decide, if vancomycin is clearly needed or if you must stop breast-feeding.
Ask your doctor or pharmacist for advice before taking this medicine.
rcj ro
—1 CD
LULU 009
2. WHAT YOU NEED TO KNOW BEFORE YOU USE VANCOMYCIN
Do not use Vancomycin
- if you are allergic to vancomycin
Warning and precautions
Talk to your doctor or pharmacist before using Vancomicin.
Before treatment with vancomycin, make sure that your doctor knows about your medical history, especially if you:
- have low blood count
- have kidney problems
- have ear problems such as deafness
- are pregnant, or planning to become pregnant
- are breastfeeding
- are elderly and over 60 years of age
- are a premature infant or a child
- are going to have surgery
In case you develop severe allergic reaction, your doctor will stop treatment with vancomycin and give you other appropriate treatment. If you will be given the infusion too fast, you can get some side effects like low blood pressure or rash. Stopping the infusion usually results in a prompt cessation of the reactions.
Vancomycin must be used with caution in patients with kidney failure or in those who receive concomitant treatment with other substances toxic to kidney as the possibility of developing toxic effects is much higher. Serial tests of kidney function should be performed and the appropriate dose regimens adhered to in order to reduce this risk.
Deafness, transitory or permanent, which may be preceeded by noises in ears, can occur in patients with prior deafness, who have received excessive doses, or who receive concomitant treatment with another substance toxic to hearing. To reduce this risk, blood levels should be determined periodically and periodic testing of hearing function is recommended.
X-----------------------------------------------
The following information is intended for medical or healthcare professionals only:
This is an extract from the Summary of Product Characteristics to assist in the administration of Vancomycin. When determining appropriateness of use in a particular patient, the prescriber should be familiar with the Summary of Product Characteristics of the medicinal product.
• Posology and method of administration
Vancomycin powder for concentrate for solution for infusion must be administered intravenously. Each dose should be administered at a rate not exceeding 10 mg/min or over a period of time of at least 60 minutes (whichever is longer).
The dose should be individually adapted according to weight, age and renal function.
The following dosage regimens are recommended:
Patients with normal renal function Adults and adolescents above 12 years of age:
The recommended daily intravenous dose is 2000 mg, divided into doses of 500 mg every 6 hours or 1000 mg every 12 hours.
For bacterial endocarditis, the generally accepted regimen is 1000 mg vancomycin intravenously every 12 hours for 4 weeks either alone or in combination with other antibiotics (gentamicin plus rifampin, gentamicin, streptomycin). Enterococcal endocarditis is treated for 6 weeks with vancomycin in combination with an aminoglycoside - according to national recommendations.
Peri-operative prophylaxis against bacterial endocarditis: Adults receive 1000 mg vancomycin intravenously prior to surgery (prior to induction of anaesthesia) and depending on time and type of surgery, the dose of 1000 mg of vancomycin IV 12 hours postoperatively can be given.
Paediatric population
Children one month to 12 years of age:
The recommended intravenous dose is 10 mg/kg, every 6 hours or 20 mg/kg every 12 hours.
Infants and newborns:
The recommended initial dose is 15 mg/kg, followed by 10 mg/kg every 12 hours during the first week of life and every 8 hours after that age and up to 1 month of age. Careful monitoring of serum concentration of vancomycin is recommended (see below).
Special population
Elderly patients:
Lower maintenance doses may be required due to the age-related reduction in renal function.
Obese patients:
Modification of the usual daily doses may be required.
Patients with hepatic insufficiency There is no evidence that the dose has to be reduced in patients with hepatic insufficiency.
Patients with impaired renal function The dose must be adjusted in patients with impaired renal function and the following nomogram can serve as guidance. Careful monitoring of serum concentration of vancomycin is recommended (see below).
Driving and using machines
Vancomycin has no or very little effect on your ability to drive and operate machines.
3. HOW TO USE VANCOMYCIN
You will be given Vancomycin by medical staff while you are in hospital.
Your doctor will decide how much of this medicine you should receive each day and how long the treatment will last.
Dosage
The dose given to you will depend on:
• your age,
• the infection you have,
• how well your kidneys are working,
• your hearing ability
• any other medicines you may be taking.
Adults and children above 12 years:
the usual dose is 2000 mg daily in two or four doses.
Children under 12 years:
will be given smaller doses, depending on their body weight.
Patients with impaired kidney function, the elderly and pre-term new-born infants: the
doctor will reduce the dose or extend the interval between two doses.
During treatment you might have blood tests, be asked to provide urine samples and possibly have hearing tests to look for signs of possible side effects.
How the treatment will be given Intravenous infusion means that the medicinal product flows from an infusion bottle or bag through a tube to one of your blood vessels and into your body. Your doctor, or nurse, will always give vancomycin into your blood, never in a muscle. Vancomycin will be diluted before being given to you, and will slowly flow into your vein for at least 60 minutes.
Creatinine clearance (ml/s)
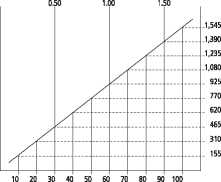
Creatinine clearance (ml/min)
Dosing nomogram for adults with impaired renal function
In patients with mild or moderate renal failure, the starting dose must not be less than 15 mg/kg. In patients with severe renal failure, it is preferable to administer a maintenance dose between 250 mg and 1000 mg at a spacing of several days rather than administer lower daily doses.
Patients with anuria (with practically no renal function) should receive doses of 15 mg/kg body weight until the therapeutic serum concentration is reached. The maintenance doses are 1.9 mg/kg body weight per 24 hours. In order to facilitate the procedure, adult patients with strongly impaired renal function may obtain a maintenance dose of 250 - 1000 mg at intervals of several days instead of a daily dose.
Dosage in case of haemodialysis
For patients without any renal function, even under
regular hemodialysis, the following dosage is also
possible:
Saturating dose 1000 mg, maintenance dose 1000 mg every 7 - 10 days.
If polysulfone membranes are used in haemodialysis (high flux dialysis), the half-life of vancomycin is reduced. An additional maintenance dose may be necessary in patients on regular haemodialysis.
Monitoring of vancomycin serum concentrations: The serum concentration of vancomycin should be monitored at the second day of treatment immediately prior to the next dose, and one hour post infusion. Therapeutic vancomycin blood levels should be between 30 and 40 mg/l (maximum 50 mg/l) one hour after the end of the infusion, the minimum level (short prior to the next administration) between 5 and 10 mg/l, or according to local recommendation.
The concentrations should normally be monitored twice or three times per week.
Method of administration:
Parenterally vancomycin shall only be administered as slow intravenous infusion (not more than 10 mg/min - over at least 60 min) which is sufficiently diluted (at least 100 ml per 500 mg or at least 200 ml per 1000 mg).
Patients requiring fluid restriction can receive a solution of 500 mg / 50 ml or 1000 mg / 100 ml. With these higher concentrations the risk for infusion related side effects can be increased.
For information about the preparation of the solution, please refer to chapter 6.6.
Duration of treatment
The duration of the treatment depends on the severity of the infection as well as on the clinical and bacteriological progress.
LULU 009
Duration of treatment
The length of treatment depends on the infection you have and may last a number of weeks.
If you take more Vancomycin than you should
As this medicine will be given to you while you are in the hospital, it is unlikely that you will be given too much vancomycin. However, tell your doctor or nurse immediately if you have any concerns.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking the medicine immediately and
seek medical attention if signs of an allergic reaction occur:
• hives; swelling of your face, lips, tongue, or throat; difficulty breathing or swallowing or dizziness.
If you think you have any of the following side effects or symptoms, tell your doctor as soon as possible:
Common side effects (affect 1 to 10 users in 100):
• decrease in blood pressure;
• swelling, redness and pain along a vein;
• breathlessness, a high pitched sound resulting from turbulent air flow in the upper airway
• generalized rash and mucosal inflammation, itching, itchy rash;
• redness of the upper body and the face, pain and contraction of the chest and back muscles;
• kidney problems which may be detected primarily by increased creatinine or urea concentrations in your blood.
Uncommon side effects (affect 1 to 10 users in 1,000):
• temporary or permanent loss of hearing.
Rare side effects (affect 1 to 10 users in 10,000):
• anaphylactic reactions, allergic reactions;
• drug fever, chills;
• increased or reduced (sometimes severely decreased) urine output, or traces of blood in urine;
• increase or decrease in some of the cells in the blood;
• noises (e.g. hissing) in ears;
• feeling faint;
• red or purple skin (possible signs of blood vessel inflammation);
• nausea.
Very rare side effects (affect less than 1 out of 10,000 patients):
Skin disorders resulting from an allergic reaction (multiple skin lesions, joint aches), cardiac arrest, or inflammation of the bowel which causes abdominal pain or bloody diarrhoea.
If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet.
5. HOW TO STORE VANCOMYCIN
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton, after EXP: {month/year}. The expiry date refers to the last day of that month.
Do not store above 25°C.
Reconstituted concentrate:
After reconstitution, the reconstituted concentrate should be diluted immediately.
Further diluted solution:
Chemical and physical in-use stability has been demonstrated for 48 hours at 2°-8°C and 25°C with Sodium Chloride 9 mg/ml (0.9%) Injection and Glucose 50 mg/ml (5%) Injection.
From a microbiological point of view, unless the method of reconstitution/dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C.
Keep the vial in the outer carton in order to protect from light.
Do not use this medicine if you notice an unclear solution and extraneous particles.
The stability of reconstituted solution is stated below in the additional information for health professionals.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Vancomycin contains
- The active substance is vancomycin (as hydrochloride).
- There are no other ingredients.
Vancomycin 500 mg powder for concentrate for solution for infusion Each vial contains:
500 mg vancomycin (as vancomycin hydrochloride) equivalent to not less than 525,000 IU.
When reconstituted with 10ml of water for injections, the resulting concentrate for solution for infusion contains 50 mg/ml vancomycin. Vancomycin 1000 mg powder for concentrate for solution for infusion Each vial contains:
1000 mg vancomycin (as vancomycin hydrochloride) equivalent to not less than 1,050,000 IU.
When reconstituted with 20 ml of water for injections, the resulting concentrate for solution for infusion contains 50 mg/ml vancomycin.
What Vancomycin looks like and contents of the pack
This medicine is a homogeneous, white to off-white freeze-dried powder for concentrate for solution for infusion. It must be first dissolved in water for injection and further diluted in an appropriate diluent prior to use.
This medicine is supplied in transparent glass vials closed with rubber stopper and sealed with aluminium and plastic flip-off caps. This medicine is available in two strengths: 500 mg and 1000 mg
Vancomycin is packed in carton boxes. Each box can contain 1 or 10 vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Auhtorization Holder Hikma Farmaceutica (Portugal), S.A.
Estrada do Rio da Mo n.° 8, 8A e 8B - Fervenga
2705-906 Terrugem SNT
Portugal
Tel.: ++351 21 960 84 10 Fax: ++351 21 961 51 02 E-mail: geral@hikma.pt
Manufacturers:
Viale Certosa, 10 27100 Pavia Italy
Tel.: +39 0382 527949 Fax: +39 0382 422745 E-mail: info@hikma.pt Hikma Farmaceutica (Portugal), S.A.
Estrada do Rio da Mo n.° 8, 8A e 8B - Fervenga
2705-906 Terrugem SNT
Portugal
Tel.: ++351 21 960 84 10 Fax: ++351 21 961 51 02 E-mail: geral@hikma.pt
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria: Vancomycin Hikma, 500 mg und
1000 mg, Pulver fur ein Konzentrat zur Herstellung einer Infusionslosung
Czech Republic: Vancomycin Hikma, 500 mg a 1000 mg, Prasek pro koncentrat pro infuzni roztok
France: Vancomycin Hikma, 500 mg et
1000 mg, Poudre pour solution a diluer pour solution pour perfusion
Ireland: Vancomycin, 500 mg and
1000 mg, Powder for concentrate for solution for infusion
Nederland: Vancomycin Hikma, 500 mg en
1000 mg, Poeder voor concentraat voor oplossing voor infusie
Portugal: Vancomicina Hikma, 500 mg e
1000 mg, Po para concentrado para solugao para perfusao
United Kingdom: Vancomycin, 500 mg and 1000 mg, Powder for concentrate for solution for infusion
X-----------------------------------------------
• Interaction with other medicinal products and other forms of interaction
Other potentially nephrotoxic or ototoxic medications
Concurrent or sequential administration of vancomycin with other potentially neurotoxic or/and nephrotoxic active substances particularly gentamicin, amphotericin B, streptomycin, neomycin, kanamycin, amikacin, tobramycin, viomycin, bacitracin, polymyxin B, colistin and cisplatin may potentiate the nephrotoxicity and/or ototoxicity of vancomycin and consequently requires careful monitoring of the patient.
Because of synergic action (e.g. with gentamicin) in these cases the maximum dose of vancomycin has to be restricted to 500 mg every 8 hours.
Anaesthetics
Concurrent administration of vancomycin and anaesthetic agents has been associated with erythema, histamine like flushing and anaphylactoid reactions. This may be reduced if the vancomycin is administered over 60 minutes before anaesthetic induction.
Muscle relaxants
If vancomycin is administered during or directly after surgery, the effect (neuromuscular blockade) of muscle relaxants (such as succinylcholine) concurrently used can be enhanced and prolonged.
• Incompatibilities
Vancomycin has a low pH that may cause chemical or physical instability when it is mixed with other substances. Therefore, each parenteral solution should be checked visually for precipitations and discolouration prior to use.
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
In case of combination therapy of vancomycin with other antibiotics/chemotherapeutics, the preparations should be administered separately. Mixtures of solutions of vancomycin and beta-lactam antibiotics have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between administration of these antibiotics. It is also recommended to dilute solutions of vancomycin to 5 mg/ml or less.
• Shelf life
Powder:
2 years
Reconstituted concentrate:
Chemical and physical in-use stability has been demonstrated for 48 hours at 2°-8°C and 25°C. After reconstitution, the reconstituted concentrate should be diluted immediately.
Further diluted solution:
Chemical and physical in-use stability has been demonstrated for 48 hours at 2°-8°C and 25°C with Sodium Chloride 9 mg/ml (0.9%) Injection and Glucose 50 mg/ml (5%) Injection.
From a microbiological point of view, unless the method of reconstitution/dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and
conditions are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C.
• Special precautions for disposal and other handling
The product must be reconstituted and the resulting concentrate must then be diluted immediately prior to use.
Preparation of the reconstituted concentrate
Dissolve Vancomycin 500 mg Powder for concentrate for solution for infusion in 10 ml of sterile Water for injection.
Dissolve Vancomycin 1000 mg Powder for concentrate for solution for infusion in 20 ml of sterile Water for injection.
One ml of reconstituted concentrate contains 50 mg of vancomycin.
Appearance of reconstituted concentrate After reconstitution the solution is clear and colourless to slightly yellowish brown without visible particles.
For storage conditions of the reconstituted medicinal product, see section 6.3.
Preparation of final diluted Solution for infusion
Reconstituted solutions containing 50 mg/ml of vancomycin should be further diluted.
Suitable diluents are:
Sodium Chloride 9 mg/ml (0.9%) Injection Glucose 50 mg/ml (5%) Injection Intermittent infusion:
Reconstituted solution containing 500 mg vancomycin (50 mg/ml) must be diluted further with at least 100 ml diluent (to 5 mg/ml). Reconstituted solution containing 1000 mg vancomycin (50 mg/ml) must be diluted further with at least 200 ml diluent (to 5 mg/ml).
The concentration of vancomycin in Solution for infusion should not exceed 5 mg/ml.
The desired dose should be administered slowly by intravenous use at a rate of no more than 10 mg/minute, for at least 60 minutes or even longer.
Continuous infusion:
This should be used only if treatment with an intermittent infusion is not possible. Dilute 1000 mg to 2000 mg of dissolved vancomycin in a sufficient amount of the above suitable diluent and administer it in the form of a drip infusion, so that the patient will receive the prescribed daily dose in 24 hours.
Appearance of diluted solution
After dilution the solution is clear, free from
extraneous particles.
For storage conditions of the diluted medicinal product, see section 6.3.
Before administration, the reconstituted and diluted solutions should be inspected visually for particulate matter and discoloration. Only clear and colourless solution free from particles should be used.
Vials are for single use only. Unused medicinal products must be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.