Vancomycin 500Mg Powder For Concentrate For Solution For Infusion
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Vancomycin 500mg Powder for Concentrate for Solution for Infusion.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains:
Vancomycin 500mg Powder for Concentrate for Solution for Infusion.
Each vial contains 500mg vancomycin (equivalent to 525,000 IU) (as vancomycin hydrochloride).
When reconstituted with 10ml of water for injections, the resulting concentrate for solution for infusion contains 50mg/ml vancomycin.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for concentrate for solution for infusion.
White to off-white powder.
Reconstituted solution pH is 2.5 - 4.5
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Intravenous vancomycin is indicated in the following severe infections caused by gram-positive bacteria susceptible to vancomycin which cannot be treated with or failed to respond or are resistant to other antibiotics such as penicillins and cephalosporins.
- endocarditis
- infections of the bones (osteomyelitis)
- pneumonia
- soft-tissue infections
Endocarditis caused by enterococci, Streptococcus viridans or S. bovis should be treated with a combination of vancomycin and an aminoglycoside.
Vancomycin may be used for the perioperative prophylaxis against bacterial endocarditis, in patients at high risk of developing bacterial endocarditis when they undergo major surgical procedures (e.g., cardiac and vascular procedures, etc) and are unable to receive a suitable beta-lactam antibacterial agent. Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Vancomycin may be used orally for the treatment of staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile. Parenteral administration of vancomycin is not effective for these indications. Intravenous administration may be used concomitantly if required.
4.2 Posology and method of administration
For intravenous infusion only.
For preparation of solution for infusion please refer to section 6.6.
Concentrations of no more than 5mg/ml are recommended. In selected patients in need of fluid restriction, a concentration up to 10mg/ml may be used; use of such higher concentrations may increase the risk of infusion-related events. Infusions should be given over at least 60 minutes. In adults, if doses exceeding 500mg are used, a rate of infusion of no more than 10mg/min is recommended. Infusion related events may occur, however, at any rate or concentration.
The dose and duration of treatment should be adjusted individually and according to the underlying type and severity of infection, and patient factors such as age and renal function.
Vancomycin levels can be measured to aid dose adjustments.
Measurement of serum concentrations
Following multiple intravenous doses, peak serum concentrations, measured two hours after infusion is complete, range from 18-26mg/l. Trough levels measured immediately prior to the next dose should be 5-10mg/l. Ototoxicity has been associated with serum drug levels of
80-100mg/l, but this is rarely seen when serum levels are kept at or below 30mg/l.
Patients with normal renal function
Adults and children above 12 years of age:
The recommended daily intravenous dose is 2000mg (2g), given as 500mg every 6 hours or 1000mg (1g) every 12 hours. Improvement is usually seen within 48 to 72 hours. The total duration of administration is determined by the type and severity of the infection and the clinical response of the patient. For bacterial endocarditis, the generally accepted regimen is 1000mg (1g) vancomycin intravenously every 12 hours for 4 weeks either alone or in combination with other antibiotics.
Longer treatment up to 6 weeks may be required, depending on the pathogen involved. National guidelines should be adhered to.
If Vancomycine is co-administrated with a aminoglycoside (e.a. gentamycine) patients should be monitored carefully for signs of neurotoxicity and ototoxicity. The dosage should be adjusted when renal disturbance occurs (see section 4.5).
Peri-operative prophylaxis against bacterial endocarditis: Adults receive 1000mg (1g) vancomycin intravenously prior to surgery (prior to induction of anaesthesia) and depending on time and type of surgery, the dose of 1000mg (1g) of vancomycin i.v. 12 hours postoperatively can be given.
Children one month to 12 years of age:
40 mg/kg/day: The dose must be divided and usually in to four doses (e.g. 10mg/kg every 6 hours). Each dose should be administered over at least 60 min.
Newborn infants (full-term):
0-7 days of age: A starting dose of 15mg/kg, followed by 10mg/kg every 12 hours.
7-30 days of age: A starting dose of 15mg/kg, followed by 10mg/kg every 8 hours.
Each dose should be administered over at least 60 min.
Close monitoring of serum vancomycin concentrations may be warranted in these patients.
The elderly:
Dosage reduction may be necessary to a greater extent than expected because of decreasing renal function (see below). Monitor auditory function, see Section 4.4.
Pregnancy:
It has been reported that significantly increased doses may be required to achieve therapeutic serum concentrations in pregnant patients.
Patients with impaired renal function
In patients with impaired renal function the dose must be adjusted to avoid toxic serum levels. Serum levels of vancomycin should be monitored regularly. For most patients with impaired renal function the following nomogramm, based on creatinine clearance, can be used to determine the dose needed.
The starting dose should always be at least 15mg/kg.
The nomogram is not valid for functionally anephric patients on dialysis.
VANCOMYCIN CLEARANCE iml.'minrttg}
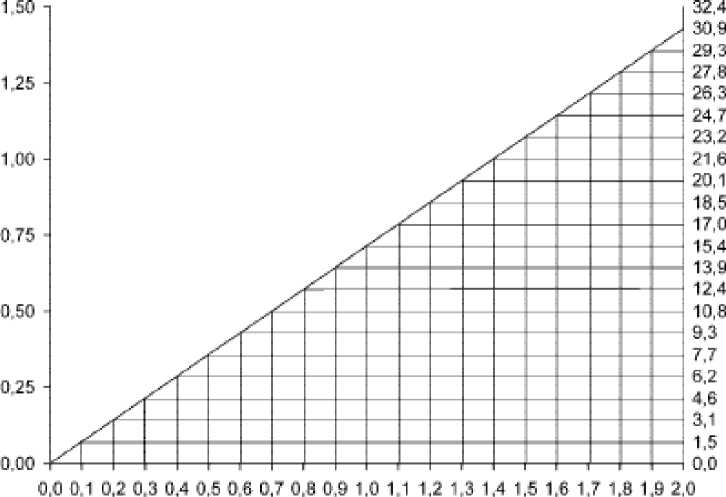
I
$
L
O
□
O
OREAT NlN CLEARANCE finl.'mirWfll
If the creatine clearance is not available, the following formula may be applied to calculate the creatinine clearance from the patient’s age, sex and serum creatinine:
Men: Weight (kg) x 140 - age (years)
72 x serum creatinine (mg/100 ml)
Women: 0.85 x value calculated by the above formula.
Where possible, the creatinine clearance should always be determined.
Patient on hemodialysis
Serum levels of vancomycin should be monitored regularly.
For anuric patients (without kidney function) on dialysis the starting dose is 15mg/kg and the maintenance dose is approximately 1.9 mg/kg/24 hours.
Since individual maintenance doses of 250mg to 1g are convenient, in patients with marked renal impairment a dose may be given every several days rather than on a daily basis. In anuria a dose of 1g every 7-10 days has been recommended
If polysulfone membranes are used for hemodialysis („high flux dialysis“), the half time of vancomycin is shortened. For patients with regular hemodialysis an additional maintenance dose may be necessary.
Patients with impaired liver function
The availability of data in patients with impaired liver function is limited. Available data do not indicate the need for dose adjustment in mild or moderate liver function impairment.
Oral administration
The contents of vials for parenteral administration may be used.
Adults and the elderly: The usual daily dose given is 500mg in divided doses for 7 to 10 days, although up to 2g/day have been used in severe cases. The total daily dosage should not exceed 2g. Each dose may be reconstituted in 30ml water and either given to the patient to drink, or administered by nasogastric tube.
Children: The usual daily dose is 40mg/kg in three or four divided doses for 7 to 10 days. The total daily dosage should not exceed 2g.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Warnings
Rapid bolus administration (eg, over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest. histamine like responses and maculopapular or erythematous rash (“red man’s syndrome” or “red neck syndrome”). Vancomycin should be infused in a dilute solution over a period of not less than 60 minutes to avoid rapid infusion-related reactions. Stopping the infusion usually results in a prompt cessation of these reactions (see Section 4.2. and Section 4.8).
In case of severe acute hypersensitivity reactions (e.g. anaphylaxis), the treatment with vancomycin has to be discontinued immediately and the usual appropriate emergency measures have to be started.
Vancomycin should be used with caution in patients with allergic reactions to teicoplanin, since cross hypersensitivity reactions between vancomycin and teicoplanin have been reported.
Vancomycin should be used with care in patients with renal insufficiency_as the possibility of developing toxic effects is much higher in the presence of prolonged high blood concentrations. The dose should be reduced according to the degree of renal impairment. The risk of toxicity is appreciably increased by high blood concentrations or prolonged therapy. Blood levels should be monitored and renal function tests should be performed regularly.
Ototoxicity, which may be transitory or permanent (see section 4.8) has been reported in patients with prior deafness, who have received excessive intravenous doses, or who receive concomitant treatment with another ototoxic active substance such as an aminoglycoside. Deafness may be preceded by tinnitus. Experience with other antibiotics suggests that deafness may be progressive despite cessation of treatment. To reduce the risk of ototoxicity, blood levels should be determined periodically and periodic testing of auditory function is recommended.
Vancomycin should be avoided in patients with previous hearing loss. If it is used in such patients, the dose should be regulated, by periodic determination of the drug level in the blood. The elderly are more susceptible to auditory damage.
Use in paediatrics: In premature neonates and young infants, it may be appropriate to confirm desired vancomycin serum concentrations.
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema and histamine-like flushing in children (see section 4.5).
Use in the elderly: The natural decrease of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted (see section 4.2).
Precautions
Vancomycin is very irritating to tissue and causes injection site necrosis if injected intramuscularly. Pain and thrombophlebitis may occur in many patients receiving vancomycin and are occasionally severe. The frequency and severity of thrombophlebitis can be minimized by administering the medicinal product slowly as a dilute solution (see section 6.6) and by changing the sites of infusion regularly.
The frequency of infusion-related reactions (hypotension, flushing, erythema, urticaria and pruritus) increases with the concomitant administration of anaesthetic agents. This may be reduced by administering the vancomycin by infusion over 60 minutes, before anaesthetic induction.
Doses should be titrated on the basis of serum levels. Blood levels should be monitored and renal function tests performed regularly.
It is a general recommendation to monitor the concentrations 2-3 times weekly.
Regular monitoring of the blood levels of vancomycin is indicated in longer-term use, particularly in patients with renal dysfunction or impaired faculty of hearing as well as in concurrent administration of nephrotoxic or ototoxic substances, respectively.
Patients with borderline renal function and individuals over the age of 60 should be given serial tests of auditory function and of vancomycin blood levels. All patients receiving the drug should have periodic haematological studies, urine analysis and renal function tests.
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin. Therefore, monitoring of serum concentrations may be appropriate in these patients.
Prolonged use of vancomycin may result in the overgrowth of non-susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis, due to C. difficile, developing in patients who received intravenous vancomycin. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea subsequent to the administration of vancomycin. Antiperistaltics are contraindicated.
Excipients:
This medicinal product contains less than 1 mmol sodium (23mg) per vial, i.e. essentially ‘sodium- free’.
4.5 Interaction with other medicinal products and other forms of interaction
Anaesthetics
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema, histamine-like flushing and anaphylactoid reactions.
Infusion-related events may be minimised by the administration of vancomycin as a
60-minute infusion prior to anaesthetic induction.
Other potentially nephtoroxic or ototoxic medicinal products Concurrent or sequential systemic or topical use of other potentially neurotoxic or nephrotoxic drugs, such as gentamycin,_amphotericin B, streptomycin, neomycin, kanamycin, amikacin, tobramycin,_bacitracin, polymixin B, colistin, viomycin or cisplatin, may potentiate the nephrotoxicity and/or ototoxicity of vancomycin and consequently requires careful monitoring. See also section 4.2 with regard to dosage adjustment in case of use with an aminoglycoside'
Muscle relaxants
There is an increased potential of neuromuscular blockade with concomitant administration of vancomycin and neuromuscular blocking agents.
4.6 Fertility, pregnancy and lactation
Pregnancy:
No sufficient safety experience is available regarding vancomycin during human pregnancy. Reproduction toxicological studies on animals do not suggest any effects on the development of the embryo, foetus or gestation period (see section 5.3).
However, vancomycin penetrates the placenta and a potential risk of embryonal and neonatal ototoxicity and nephrotoxicity cannot be excluded. Therefore vancomycin should be given in pregnancy only if clearly needed and after a careful risk/benefit evaluation.
Lactation:
Vancomycin is excreted in human milk. Caution should be excercised when given to breast-feeding mothers because of potential adverse reactions in the infant (disturbances in the intestinal flora with diarrhoea, colonisation with yeast-like fungi and possibly sensibilisation).
Considering the importance of this medicine for nursing mother, the decision to stop breastfeeding should be considered.
4.7 Effects on ability to drive and use machines
Vancomycin has negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
The adverse reactions listed below is defined using the following MedDRA
convention and system organ class database:
very common (> 1/10);
common (> 1/100 to < 1/10);
uncommon (> 1/1,000 to < 1/100);
rare (> 1/10,000 to < 1/1,000);
very rare (< 1/10,000),
not known (cannot be estimated from the available data).
The most common adverse reactions are phlebitis and pseudo-allergic reactions in connection with too rapid intravenous infusion of vancomycin.
Infusion related events:
During or shortly after rapid infusion anaphylactoid reactions may occur, including hypotension, dyspnea, urticaria or pruritus. Redness of the skin on the upper body (Red man syndrome), pain and cramps in chest or back muscle can occur.
The reactions abate when administration is stopped, generally between 20 minutes and
2 hours. Vancomycin should be infused slowly (for more than 60 minutes - see section 4.4).
Otoxicity has primarily been reported in patients given high doses, or concomitant treatment with other ototoxic medicinal product, or had a preexisting reduction in kidney function or hearing.
Blood and the lymphatic system disorder:
Rare: Thrombocytopenia, neutropenia, agranulocytosis, eosinophilia..
Immune system disorders
Rare: Anaphylactic reactions, hypersensitivity reactions.
Ear and labyrinth disorders:
Uncommon: Transient or permanent loss of hearing.
Rare: Tinnitus, dizziness.
Cardiac disorders:
Very rare: Cardiac arrest.
Vascular disorders:
Common: Decrease in blood pressure, thrombophlebitis.
Very rare: Vasculitis
Respiratory, thoracic and mediastinal disorders:
Common: Dyspnoea, stridor.
Gastrointestinal disorders:
Rare: Nausea, diarrhoea
Very rare: Pseudomembranous enterocolitis.
Skin and subcutaneous tissue disorders:
Common: Exanthema and mucosal inflammation, pruritus, urticaria.
Very rare: Exfoliative dermatitis, Stevens-Johnson syndrome, linear IgA bullous dermatosis, Lyell's syndrome.
Renal and urinary disorders:
Common: Renal insufficiency manifested primarily by increased serum
creatinine.
Rare: Interstitial nephritis, acute renal failure.
General disorders and administration site conditions:
Common: Phlebitis, redness of the upper body and the face, pain and
spasm of the chest and back muscles Rare: Drug fever, shivering.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome) has been reported during postmarketing experience.
4.9 Overdose
Toxicity due to overdose has been reported. 500mg IV to a child, 2 year of age, resulted in lethal intoxication. Administration of a total of 56g during 10 days to an adult resulted in renal insufficiency. In certain high-risk conditions (e. g. in case of severe renal impairment) high serum levels and oto- and nephrotoxic effects can occur.
Measures in case of overdose
• A specific antidote is not known.
• Symptomatic treatment while maintaining renal function is required.
• Vancomycin is poorly removed from the blood by haemodialysis or peritoneal dialysis. Haemofiltration or haemoperfusion with polysulfone resins have been used to reduce serum concentrations of vancomycin.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Glycopeptide Antibacterials, ATC code: J01X A01
Mode of action
Vancomycin is a tricyclic glycopeptide antibiotic that inhibits the synthesis of the cell wall in sensitive bacteria by binding with high affinity to the D-alanyl-D-alanine terminus of cell wall precursor units. The drug is bactericidal for dividing microorganisms.
PK/PD relationship
Vancomycin activity is considered to be time-dependent - that is, antimicrobial activity depends on the duration that the drug level exceeds the minimum inhibitory concentration (MIC) of the target organism.
Mechanism of resistance:
Acquired resistance to glycopeptides is based on acquisition of various van gene complexes and alteration of the D-alanyl-D-alanine target to D-alanyl-D-lactate or D-alanyl-D-serine which bind vancomycin poorly, because a critical site for hydrogen bonding is missing. This form of resistance is especially seen in Enterococcus faecium.
The reduced susceptibility or resistance to vancomycin in Staphylococcus is not well understood. Several genetic elements and multiple mutations are required.
Cross-resistance with teicoplanin has been reported.
Susceptibility:
Vancomycin is active against gram-positive bacteria. Gram-negative bacteria are resistant.
The MIC breakpoints separating susceptible from resistant organisms are as follows:
EUCAST (European Committee on Antimicrobial Susceptibility Testing) recommendations
|
Susceptible |
Resistant | |
|
Staphylococcus spp. |
<2 mg/L |
>2 mg/L |
|
Enterococcus spp. |
<4 mg/L |
> 4 mg/L |
|
Streptococcus spp |
< 2 mg/L |
> 2 mg/L |
|
Streptococcus pneumoniae |
< 2 mg/L |
> 2 mg/L |
|
Gram-positive anaerobes |
< 2 mg/L |
< 2 mg/L |
|
Non species related* |
< 2 mg/L |
> 4 mg/L |
|
* Non-species related breakpoints have been c |
etermined mainly | |
of PK/PD data and are independent of MIC distributions of specific species. They are for use only for species that have not been given a species-specific breakpoint and not for those species where susceptibility testing is not recommended.
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Classes_
Commonly susceptible species Gram positive
Enterococcus faecalis.
Staphylococcus aureus Staphylococcus coagulase negative Streptococcus spp.
Streptococcus pneumoniae
Clostridium spp._
Species for which acquire d resistance may be a problem
Enterococcus faecium_
Inherently resistant
Gram negative bacteria Chlamydia spp.
Mycobacteria Mycoplasma spp.
Rickettsia spp._
5.2 Pharmacokinetic properties
Vancomycin appears in various body fluids, including pleural, pericardial, synovial and ascetic fluids.
A single intravenous dose of 1g in adults produces plasma concentrations of 15 to 30pg/ml
1 hour after 1- to 2-hour infusion.
Vancomycin is metabolized only to a low extent. After parenteral administration it is excreted almost completely as microbiologically active substance (approx. 75-90% within 24 hours) through glomerular filtration via the kidneys. Biliary excretion is insignificant (less than 5% of a dose).
The serum elimination half-life is about 4-6 hours in adults with normal renal-function and 2,2-3 hours in children. In patients with impaired renal function the serum elimination half-life can be significantly increased (up to 7.5 days). The total systematic and renal clearance of vancomycin may be reduced in the elderly due to the natural decrease in glomerular filtration.
The volume of distribution is 0.4-1 L/kg. The binding of vancomycin to protein has been reported in the literature to range from 10% to 50%. Factors that affect the overall activity of vancomycin include its tissue distribution, inoculum size, and protein-binding effects.
Vancomycin is not significantly absorbed from the normal gastro-intestinal tract and is therefore not effective by the oral route for infections other than staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile.
Orally administered vancomycin does not usually enter the systemic circulation even when inflammatory lesions are present. Measurable serum concentrations may occur infrequently in patients with active C. difficile -induced pseudomembranous colitis and, in the presence of renal impairment, the possibility of accumulation exists.
Administration of vancomycin oral solution, 2g daily for 16 days to anephric patients with no inflammatory bowel disease, gave serum levels of <0.66g/ml. With doses of 2g daily, concentration of 3,100mg/kg can be found in the faeces and levels of <1g/ml can be found in serum of patients with normal renal function who have pseudomembranous colitis1.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity.
Limited data on mutagenic effects show negative results, long-term studies in animals regarding a carcinogenic potential are not available.
In teratogenicity studies, where rats and rabbits received doses approximately corresponding to the human dose based on body surface (mg/m2), no direct or indirect teratogenic effects were observed.
Animal studies of the use during the perinatal/postnatal period and regarding effects on fertility are not available.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Sodium hydroxide (for pH adjustment) Hydrochloric acid (for pH adjustment)
6.2 Incompatibilities
Vancomycin has a low pH that may cause chemical or physical instability when it is mixed with other compounds. Mixing with alkaline solutions should be avoided. Therefore, each parenteral solution should be checked visually for precipitation and discolouration prior to use.
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life
Shelf life of powder as packaged for sale: 2 years
Shelf-life of reconstituted concentrate: The reconstituted concentrate should be diluted immediately after preparation. For oral use the reconstituted concentrate should be used immediately.
Shelf-life of diluted product:
Chemical and physical in-use stability of the diluted product has been demonstrated for 48 hours at both 2-8°C and 25°C when diluted with either 0.9% sodium chloride or 5% glucose.
From a microbiological point of view, the medicinal product should be used immediately_unless reconstitution and dilution has taken place in controlled and validated aseptic conditions.
If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and the product should be protected from light during storage.
6.4 Special precautions for storage
Powder as packed for sale Store below 25°C.
Keep the vial in the outer carton in order to protect from light
For storage conditions of the reconstituted medicinal product and diluted product, see section 6.3.
Solutions of the parenteral powder intended for oral administration may be stored in a refrigerator (2-8 °C) for 96 hours.
6.5 Nature and contents of container
Vancomycin hydrochloride 500mg:
Powder in a glass vial (type I) closed with a rubber stopper (bromobutyl rubber) and aluminium seal with flip off cap.
Pack size: 1 and 10 vials in a carton
6.6 Special precautions for disposal
The powder must be reconstituted and the resulting concentrate must then be immediately diluted further prior to use
Preparation of the reconstituted concentrate
Dissolve the content of each 500mg vial in 10ml of sterile water for injections.
One ml of reconstituted solution contains 50mg of vancomycin. pH of the reconstituted solution is 2.5 to 4.5.
Appearance of reconstituted solution
Clear colourless to pale yellow solution free from fibre and visible particulate matters For storage conditions of the reconstituted medicinal product, see sections 6.3 Preparation of final diluted Solution for infusion
Reconstituted solutions containing 50mg/ml of vancomycin should be further diluted depending on the method of administration.
Suitable diluents are:
5% Glucose Injection
0.9% Sodium Chloride Injection
Intermittent infusion:
Reconstituted solution containing 500mg of vancomycin (50mg/ml) must be diluted further with at least 100ml diluent.
The concentration of vancomycin in Solution for infusion should not exceed 5mg/ml.
The desired dose should be administered slowly by intravenous infusion at a rate of no more than 10mg/minute, for at least 60 minutes or even longer.
For storage conditions of the diluted medicinal product, see sections 6.3
Before administration, the reconstituted and diluted solutions should be inspected visually for particulate matter and discoloration. Only clear and colourless to pale yellow solution free from particles should be used.
Disposal
Vials are for single use only. Unused product must be discarded.
Any unused product or waste material should be disposed of in accordance with local requirements.
Oral administration
The contents of vials for parenteral administration may be used.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
MARKETING AUTHORISATION HOLDER
7
Actavis Group PTC ehf. Reykjavikurvegur 76-78, 220 HafnarfjorQur Iceland
8 MARKETING AUTHORISATION NUMBER(S)
PL 30306/0444
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
15/08/2011
10 DATE OF REVISION OF THE TEXT
02/07/2013