Versatis 5% Medicated Plaster
Versatis® 5% medicated plaster
_(lidocaine)_
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.



• close the sachet tightly after use
• the plaster contains water, and will dry out if the sachet is not closed properly.
liner
PACKAGE LEAFLET: INFORMATION FOR THE USER
Your medicine is available using the name Versatis 5% medicated plaster but will be referred to as Versatis throughout this leaflet.
What is in this leaflet:
1. What Versatis is and what it is used for
2. What you need to know before you use Versatis
3. How to use Versatis
4. Possible side effects
5. How to store Versatis
6. Contents of the pack and other information
1. WHAT VERSATIS IS AND WHAT IT IS USED FOR
Versatis contains lidocaine, a local analgesic, which works by reducing the pain in your skin.
You have been given Versatis to treat a painful skin condition called post-herpetic neuralgia. This is generally characterised by localised symptoms such as burning, shooting or stabbing pain.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE VERSATIS
Do not use Versatis
• if you are allergic to lidocaine or any of the other ingredients of this medicine (listed in section 6).
• if you have had an allergic reaction to other products which are similar to lidocaine, such as bupivacaine, etidocaine, mepivacaine or prilocaine.
• on injured skin or open wounds.
Warnings and precautions
Talk to your doctor or pharmacist before using Versatis.
If you have severe liver disease, or severe heart problems, or severe kidney problems, you should talk to your doctor before using Versatis. Versatis should only be used on the areas of skin after the shingles has healed. It should not be used on or near the eyes or mouth. Lidocaine is broken down in your liver to several compounds.
One of these compounds is 2,6 xylidine which has been shown to cause tumours in rats when given lifelong in very high doses. The significance of these findings in humans is not known.
Children and adolescents
Versatis has not been studied in patients under 18 years of age. Therefore it is not recommended for use in this patient population.
Other medicines and Versatis
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Versatis should not be used in pregnancy unless clearly necessary.
There are no studies of the plaster in breast-feeding women. When using Versatis, only very small amounts of the active substance lidocaine may be present in the blood stream. An effect on breast-fed infants is unlikely.
Driving and using machines
An effect of Versatis on the ability to drive and use machines is unlikely. Therefore you may drive or operate machinery whilst using Versatis.
Versatis contains propylene glycol, methyl parahydroxybenzoate and propyl parahydroxybenzoate
The plasters contain propylene glycol (E1520) which may cause skin irritation. In addition it contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions. The allergic reactions may sometimes occur after you have been using the plaster for some time.
3. HOW TO USE VERSATIS
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The usual daily dose is to use between one and three plasters of the size of the painful areas of your skin. Versatis may be cut into smaller pieces to fit the affected area. You should not use more than 3 plasters at the same time.
The plasters should be removed after 12 hours of use, so that you have a 12 hour period with no plaster.
You can choose to apply Versatis during the day or during the night.
Usually, you will feel some pain relief on the first day you use the plaster, but it may take up to 2 - 4 weeks until the full pain-relief effect of Versatis is seen. If after that time you still have a lot of pain, please talk to your doctor because the benefits of the treatment must be weighed against potential risks (see Section 2 under 'Warnings and precautions').
Your doctor will check how well Versatis is working at regular intervals.
Before you stick Versatis on the affected area
• If the painful area of skin has hairs on it, you can cut the hairs off using scissors. Do not shave them off.
• The skin should be clean and dry.
• Creams and lotions may be used on the affected skin during the period when you are not wearing the plaster.
• If you have had a recent bath or shower, you should wait until your skin cools before using the plaster.
Sticking the plaster on
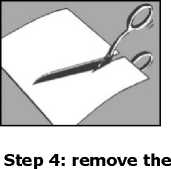
Step 1: open the sachet and remove one or more plasters
• tear open or cut the sachet along the dotted line
• when using scissors, be careful not to damage the plasters
• take out one or more plasters depending on the size of the painful area on your skin Step 2: close the sachet
Step 3: cut the plaster, if necessary • if required, cut the plaster to the required size to fit the painful area of skin before removing the liner.
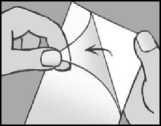
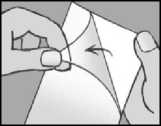
remove the transparent liner from the plaster try not to touch the sticky part of the plaster
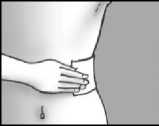
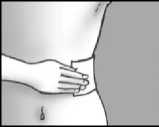
Step 5: apply the plaster and press it firmly onto the skin
apply up to three plasters to the painful area of skin press the plaster onto your skin
press for at least 10 seconds to make sure the plaster sticks firmly make sure that all of it sticks to your skin, including the edges
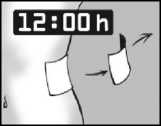
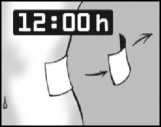
Leave the plaster on for 12 hours only
It is important that Versatis is in contact with your skin for only 12 hours. For example, if you have more pain at night you might want to apply the plaster at 7pm in the evening and remove it at 7am in the morning.
If you have more pain during the day than at night you might want to apply Versatis at 7am in the morning, and remove it at 7pm in the evening.
Bathing, showering and swimming
If at all possible contact with water should be avoided whilst using Versatis. Bathing, showering or swimming can be done in the time period when you are not wearing the plaster. If you have had a recent bath or shower, you should wait until your skin cools before using the plaster.
If the plaster comes off
Very rarely the plaster might fall off, or come unstuck. If it does, try sticking it back on the same area. If it does not stay on, remove it and put a new plaster on the same area.
How to remove Versatis
When changing the plaster, remove the old plaster slowly. If it does not come off easily, you can soak it in warm water for a few minutes before removing the plaster.
If you forget to remove the plaster after 12 hours
As soon as you remember, remove the old plaster. A new plaster can be used again after 12 hours.
If you use more plasters than you should
If you use more plasters than necessary or wear them for too long, this may increase the risk of getting side effects.
If you forget to use Versatis
After the 12 hour period with no plaster, if you have forgotten to use a new plaster, you should stick on a new plaster as soon as you remember.
If you have any further questions on use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If irritation or burning sensation occurs whilst you are using the plaster, the plaster should be removed. The area of irritation should remain plaster free until the irritation stops.
Very common side effects which may affect more than 1 in 10 people are listed below.
These include skin conditions at or around the site of plaster application, which may include redness, rash, itching, burning, dermatitis, and small blisters.
Uncommon side effects which may affect up to 1 in 100 people are listed below.
Skin injury and skin wounds.
Very rare side effects which may affect up to 1 in 10,000 people are listed below.
Open wound, severe allergic reaction and allergy.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Leaflet issue and revision date (ref): 24.06.16 Versatis® is a registered trademark of Grunenthal GmbH.
Other formats:
To listen to or request a copy of this leaflet in Braille, large font or audio please contact 01302 365000 and ask for the Regulatory Department.
Please be ready to give the following information:
Product name Versatis 5% medicated plaster
Reference number 21828/0684
POM
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE VERSATIS
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the sachet and carton after EXP. The expiry date refers to the last day of that month.
Do not refrigerate or freeze.
After first opening: keep the sachet tightly closed.
After opening the sachet, the plasters should be used within 14 days.
Do not use this medicine if you notice that the sachet has been damaged. If this has occurred, the plasters may dry out and become less sticky.
How to throw away Versatis
Used plasters still contain active ingredient, which may be harmful to others. Fold the used plasters in half, with the sticky sides together and throw them away so that they are out of the reach of children.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Versatis contains
The active substance is lidocaine.
Each 10cm x 14cm plaster contains 700mg (5% w/w) lidocaine.
The other ingredients in the plaster are glycerol, sorbitol liquid crystallising, carmellose sodium, propylene glycol (E1520), urea, heavy kaolin, tartaric acid, gelatin, polyvinyl alcohol, aluminium glycinate, disodium edetate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), polyacrylic acid, sodium polyacrylate and purified water.
Backing fabric and release liner: polyethylene terephthalate (PET).
What Versatis looks like and contents of the pack
The medicated plaster is 14cm long and 10cm wide.
White, hydrogel plaster containing adhesive material, which is applied to a non-woven polyethylene terephthalate backing embossed with "Lidocaine 5%" and covered with a polyethylene terephthalate film release liner in a re-sealable paper/foil sachet.
Each carton contains 30 plasters packed in 6 re-sealable sachets.
Each sachet contains 5 plasters.
Manufacturer:
Manufactured by: Grunenthal GmbH, Zieglerstrasse 6, 52078 Aachen, Germany.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR, UK.
Product Licence holder: Landmark Pharma Ltd., 7 Regents Drive, Prudhoe, Northumberland, NE42 6PX.
PL: 21828/0684
Lidocaine 5% medicated plaster
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.


• close the sachet tightly after use
• the plaster contains water, and will dry out if the sachet is not closed properly.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Your medicine is available using the name Lidocaine 5% medicated plaster but will be referred to as Lidocaine throughout this leaflet.
What is in this leaflet:
1. What Lidocaine is and what it is used for
2. What you need to know before you use Lidocaine
3. How to use Lidocaine
4. Possible side effects
5. How to store Lidocaine
6. Contents of the pack and other information
1. WHAT LIDOCAINE IS AND WHAT IT IS USED FOR
Lidocaine contains lidocaine, a local analgesic, which works by reducing the pain in your skin.
You have been given Lidocaine to treat a painful skin condition called post-herpetic neuralgia. This is generally characterised by localised symptoms such as burning, shooting or stabbing pain.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE LIDOCAINE Do not use Lidocaine
• if you are allergic to lidocaine or any of the other ingredients of this medicine (listed in section 6).
• if you have had an allergic reaction to other products which are similar to lidocaine, such as bupivacaine, etidocaine, mepivacaine or prilocaine.
• on injured skin or open wounds.
Warnings and precautions
Talk to your doctor or pharmacist before using Lidocaine.
If you have severe liver disease, or severe heart problems, or severe kidney problems, you should talk to your doctor before using Lidocaine. Lidocaine should only be used on the areas of skin after the shingles has healed. It should not be used on or near the eyes or mouth.
Lidocaine is broken down in your liver to several compounds.
One of these compounds is 2,6 xylidine which has been shown to cause tumours in rats when given lifelong in very high doses. The significance of these findings in humans is not known.
Children and adolescents
Lidocaine has not been studied in patients under 18 years of age. Therefore it is not recommended for use in this patient population.
Other medicines and Lidocaine
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Lidocaine should not be used in pregnancy unless clearly necessary.
There are no studies of the plaster in breast-feeding women. When using Lidocaine, only very small amounts of the active substance lidocaine may be present in the blood stream. An effect on breast-fed infants is unlikely.
Driving and using machines
An effect of Lidocaine on the ability to drive and use machines is unlikely. Therefore you may drive or operate machinery whilst using Lidocaine.
Lidocaine contains propylene glycol, methyl parahydroxybenzoate and propyl parahydroxybenzoate
The plasters contain propylene glycol (E1520) which may cause skin irritation. In addition it contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions. The allergic reactions may sometimes occur after you have been using the plaster for some time.
3. HOW TO USE LIDOCAINE
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The usual daily dose is to use between one and three plasters of the size of the painful areas of your skin. Lidocaine may be cut into smaller pieces to fit the affected area. You should not use more than 3 plasters at the same time.
The plasters should be removed after 12 hours of use, so that you have a 12 hour period with no plaster.
You can choose to apply Lidocaine during the day or during the night.
Usually, you will feel some pain relief on the first day you use the plaster, but it may take up to
2 - 4 weeks until the full pain-relief effect of Lidocaine is seen. If after that time you still have a
lot of pain, please talk to your doctor because the benefits of the treatment must be weighed
against potential risks (see Section 2 under 'Warnings and precautions').
Your doctor will check how well Lidocaine is working at regular intervals.
Before you stick Lidocaine on the affected area
• If the painful area of skin has hairs on it, you can cut the hairs off using scissors. Do not shave them off.
• The skin should be clean and dry.
• Creams and lotions may be used on the affected skin during the period when you are not wearing the plaster.
• If you have had a recent bath or shower, you should wait until your skin cools before using the plaster.
Sticking the plaster on
Step 1: open the sachet and remove one or more plasters

• tear open or cut the sachet along the dotted line
• when using scissors, be careful not to damage the plasters
• take out one or more plasters depending on the size of the painful area on your skin Step 2: close the sachet
Step 3: cut the plaster, if necessary

remove the transparent liner from the plaster try not to touch the sticky part of the plaster
Step 5: apply the plaster and press it firmly onto the skin
apply up to three plasters to the painful area of skin press the plaster onto your skin
press for at least 10 seconds to make sure the plaster sticks firmly make sure that all of it sticks to your skin, including the edges
Leave the plaster on for 12 hours only
It is important that Lidocaine is in contact with your skin for only 12 hours. For example, if you have more pain at night you might want to apply the plaster at 7pm in the evening and remove it at 7am in the morning.
If you have more pain during the day than at night you might want to apply Lidocaine at 7am in the morning, and remove it at 7pm in the evening.
Bathing, showering and swimming
If at all possible contact with water should be avoided whilst using Lidocaine. Bathing, showering or swimming can be done in the time period when you are not wearing the plaster. If you have had a recent bath or shower, you should wait until your skin cools before using the plaster.
If the plaster comes off
Very rarely the plaster might fall off, or come unstuck. If it does, try sticking it back on the same area. If it does not stay on, remove it and put a new plaster on the same area.
POM
How to remove Lidocaine
When changing the plaster, remove the old plaster slowly. If it does not come off easily, you can soak it in warm water for a few minutes before removing the plaster.
If you forget to remove the plaster after 12 hours
As soon as you remember, remove the old plaster. A new plaster can be used again after 12 hours.
If you use more plasters than you should
If you use more plasters than necessary or wear them for too long, this may increase the risk of getting side effects.
If you forget to use Lidocaine
After the 12 hour period with no plaster, if you have forgotten to use a new plaster, you should stick on a new plaster as soon as you remember.
If you have any further questions on use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If irritation or burning sensation occurs whilst you are using the plaster, the plaster should be removed. The area of irritation should remain plaster free until the irritation stops.
Very common side effects which may affect more than 1 in 10 people are listed below.
These include skin conditions at or around the site of plaster application, which may include redness, rash, itching, burning, dermatitis, and small blisters.
Uncommon side effects which may affect up to 1 in 100 people are listed below.
Skin injury and skin wounds
Very rare side effects which may affect up to 1 in 10,000 people are listed below.
Open wound, severe allergic reaction and allergy.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE LIDOCAINE
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the sachet and carton after EXP. The expiry date refers to the last day of that month.
Do not refrigerate or freeze.
After first opening: keep the sachet tightly closed.
After opening the sachet, the plasters should be used within 14 days.
Do not use this medicine if you notice that the sachet has been damaged. If this has occurred, the plasters may dry out and become less sticky.
How to throw away Lidocaine
Used plasters still contain active ingredient, which may be harmful to others. Fold the used plasters in half, with the sticky sides together and throw them away so that they are out of the reach of children.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Lidocaine contains
The active substance is lidocaine.
Each 10cm x 14cm plaster contains 700mg (5% w/w) lidocaine.
The other ingredients in the plaster are glycerol, sorbitol liquid crystallising, carmellose sodium, propylene glycol (E1520), urea, heavy kaolin, tartaric acid, gelatin, polyvinyl alcohol, aluminium glycinate, disodium edetate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), polyacrylic acid, sodium polyacrylate and purified water.
Backing fabric and release liner: polyethylene terephthalate (PET).
What Lidocaine looks like and contents of the pack
The medicated plaster is 14cm long and 10cm wide.
White, hydrogel plaster containing adhesive material, which is applied to a non-woven polyethylene terephthalate backing embossed with "Lidocaine 5%" and covered with a polyethylene terephthalate film release liner in a re-sealable paper/foil sachet.
Each carton contains 30 plasters packed in 6 re-sealable sachets.
Each sachet contains 5 plasters.
Manufacturer:
Manufactured by: Grunenthal GmbH, Zieglerstrasse 6, 52078 Aachen, Germany.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR, UK.
Product Licence holder: Landmark Pharma Ltd., 7 Regents Drive, Prudhoe, Northumberland, NE42 6PX.
PL: 21828/0684
Leaflet issue and revision date (ref): 24.06.16 Other formats:
To listen to or request a copy of this leaflet in Braille, large font or audio please contact 01302 365000 and ask for the Regulatory Department.
Please be ready to give the following information:
Product name Lidocaine 5% medicated plaster
Reference number 21828/0684
Page 2 of 2