Vertine Cfc-Free Inhaler 25 Micrograms Per Actuation Pressurised Inhalation Suspension
|
TPWT |
Ref: 231-30-50011-E LEA SALMETEROL 25mcg INH TUK<CIP |
28 May 2013 | ||||
|
TEVA UK LIMITED |
Version: 1 |
Trackwise Parent: |
Child: | |||
PL Number(s), PL 00289/1667. TEVA UK Limited Licence, Teva Regulatory Team.
MA Holder & Packer: Packed at Cipla Limited.

F. P. Code: 231-10-01777
EAN Code:
Pharma Code: Added by 3rd Party.
Edge Code:
Third party code:
Fonts: Univers
Base Font Size: 9.5 Pt
Dimensions:
L:
W:
D:
Foil Width: Perforated:
390 mm 240 mm
Colours:
(PANTON E® is a registered trademark of Pantone, Inc.)
PANTONE® GREEN C BLACK
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.
>
390mm
E
E
lo
CN
no


PACKAGE LEAFLET: INFORMATION FORTHE USER
Vertine® CFC-free Inhaler 25 micrograms per actuation pressurised inhalation, suspension
salmeterol (as xinafoate)
The name of this medicine is Vertine® CFC-free Inhaler 25 micrograms per
actuation pressurised inhalation suspension which will be referred to as
Vertine® Inhaler throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, asthma nurse or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, asthma nurse or pharmacist.
IN THIS LEAFLET:
1. What Vertine® Inhaler is and what it is used for
2. Before you use Vertine® Inhaler
3. Howto use Vertine® Inhaler
4. Possible side effects
5. Howto store Vertine® Inhaler
6. Further information
OWHAT VERTINE® INHALER IS AND WHAT IT IS USED FOR
• Vertine® Inhaler contains the medicine salmeterol. It is a long-acting bronchodilator. It helps the airways in the lungs to stay open.This makes it easier for airto get in and out.The effects can usually be noticed within 10 to 20 minutes and last for 12 hours or more.
• The doctor has prescribed Vertine® Inhaler to help prevent breathing problems. These could be caused by asthma. Taking Vertine® Inhaler regularly will prevent asthma attacks including asthma brought on by exercise or at night.
• Taking Vertine® Inhaler regularly will also help prevent breathing problems caused by other chest illnesses such as chronic obstructive pulmonary disease (COPD).
• Vertine® Inhaler helps to stop breathlessness and wheezing coming on. It does not work once you are breathless or wheezy. If that happens you need to use a fast-acting "reliever" medicine, such as salbutamol.
• Your medicine is supplied to you in an inhaler. You breathe the medicine through your mouth directly into your lungs.
• Vertine® Inhaler contains norflurane and does not contain any chlorofluorocarbons (CFCs). Norflurane-containing inhalers are less harmful to the environment than older CFC-containing inhalers. Vertine® Inhaler may taste differently to older CFC-containing inhalers. This will make no difference to howyour medicine works.
If you are being treated for asthma, you should always be given both a
Vertine® Inhaler and a steroid inhaler (or rarely steroid tablets) to use
together. Both inhalers must be used regularly.
BEFORE YOU USE VERTINE® INHALER
Do not use Vertine® Inhaler
• If you are allergic (hypersensitive) to salmeterol xinafoate or any of the other ingredients in this medicine.
• If you are allergic to peanut or soya, do not use Vertine® Inhaler as it contains soya lecithin.
Take special care with Vertine® Inhaler
• If your asthma or breathing gets worse tell your doctor straight away.
You may find that you feel more wheezy, your chest feels tight more often or you may need to use more of your fast-acting "reliever" medicine. If any of these happen, do not increase your number of puffs of Vertine® Inhaler. Your chest condition may be getting worse and you could become seriously ill. See your doctor or asthma nurse as you may need a change in asthma treatment.
• If you have been prescribed Vertine® Inhaler for your asthma, continue to use any other asthma medication you are already taking including your steroid inhaler or steroid tablets. Continue taking the same doses as before, unless your doctor tells you otherwise. Do this even if you feel much better. Do not stop using your steroid inhaler (or taking any steroid tablets) when you start using Vertine® Inhaler.
• Your doctor may want to check your health more regularly if you have an overactive thyroid gland, heart disease, including an irregular or fast heartbeat or diabetes mellitus (as salmeterol may increase your blood sugar). If you have diabetes your doctor may want to monitor your blood sugar more frequently than usual and may need to adjust the treatment you take for your diabetes.
Taking other medicines
Please tell your doctor, pharmacist or asthma nurse if you are taking or have recently taken any other medicines, including asthma medicines and any inhalers, and including medicines obtained without a prescription.This is because Vertine® Inhaler may not be suitable to be taken with other medicines.
• Please inform your doctor or asthma nurse before using Vertine® Inhaler if you are currently being treated for any fungal infections with medicines containing ketoconazole or itraconazole, or if you are being treated for HIV with ritonavir. These medicines may increase the risk of you experiencing side effects with salmeterol, including irregular heart beats, or may make side effects worse.
• Beta blockers should be avoided when using Vertine® Inhaler, unless your doctor tells you to take them. Beta blockers, including atenolol, propranolol and sotalol, are mostly used for the treatment of high blood pressure or other heart conditions. Please tell your doctor or asthma nurse if you are taking p blockers or have recently been prescribed p blockers as they may reduce or abolish the effects of salmeterol.
• Salmeterol can reduce the amount of potassium in your blood. If this happens, you may notice an uneven heartbeat, muscle weakness or cramp.This is more likely to happen if you use salmeterol with some medicines used to treat high blood pressure (diuretics) and other medicines used to treat breathing problems such as theophylline or steroids. Your doctor may askyou to have blood tests to check the amount of potassium in your blood from time to time. If you have any concerns discuss them with your doctor or asthma nurse.
Pregnancy and breast-feeding
If you are pregnant, thinkyou might be pregnant, planning to get pregnant or breast-feeding, you should talk to your doctor or asthma nurse before starting to use Vertine® Inhaler. They will assess if you can use Vertine® Inhaler during this time.
Driving and using machines
The possible side effects associated with Vertine® Inhaler are unlikely to affect your ability to drive or use machines.
HOWTO USE VERTINE® INHALER
• If you are being treated for asthma, you should always be given both a Vertine® Inhaler and a steroid inhaler to use together.
• Use Vertine® Inhaler every day until your doctor advises you to stop.
• You will start to feel your medicine working within the first day of use.
Vertine® Inhaler should be inhaled through the mouth.
Children
The safety and efficacy of Vertine® Inhaler have not been demonstrated in children. Therefore Vertine® Inhaler should not be used in children 12 years of age and younger.
Dosage for adults and adolescents over 12 years of age with asthma
• The usual starting dose is 2 puffs twice a day.
• If you have more severe asthma, your doctor may increase your dose to 4 puffs twice a day.
Dosage for adults with chronic obstructive pulmonary disease (COPD) including bronchitis and emphysema
• The usual starting dose is 2 puffs twice a day.
• Not for use in children and adolescents.
Instructions for use
Your doctor, asthma nurse or pharmacist should show you how to use your inhaler.They should check howyou use it from time to time. Incorrect use, not using the inhaler as prescribed or not using it as described in this Leaflet, may mean that the medicine will not work as it should and so will not help your asthma or COPD.
The medicine is contained in a pressurised canister in a plastic casing with a
mouthpiece.
Testing your inhaler
When you use the inhaler for the first time you should test if it works properly.
1. Remove the mouthpiece cover by gently pressing the sides with your thumb and index finger.
2. Shake the inhaler, point the mouthpiece away from you and press the canister twice to release two puffs into the air. If you have not used the inhalerfor a week or more or if you have cleaned the inhaler, you should release one puff into the air.
Y
E
E
lo
co
|
k | |||
|
PHARMACODE |
V3idV | ||
|
AREA |
3QODVIAiyVHd | ||
|
r |
>
30mm
|
TrIW7 |
Ref: 231-30-50011-E LEA SALMETEROL 25mcg INH TUK <CIP |
28 May 2013 | ||||
|
TEVA UK LIMITED |
Version: 1 |
Trackwise Parent: |
Child: | |||
PL Number(s), PL 00289/1667. TEVA UK Limited Licence, Teva Regulatory Team.
MA Holder & Packer: Packed at Cipla Limited.
F. P. Code: 231-10-01777
EAN Code:
Pharma Code: Added by 3rd Party.
Edge Code:
Third party code:
Fonts: Univers
Base Font Size: 9.5 Pt
Dimensions:
L:
W:
D:
Foil Width: Perforated:
390 mm 240 mm
Colours:
(PANTON E® is a registered trademark of Pantone, Inc.)
PANTONE® GREEN C BLACK
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.

Using your inhaler
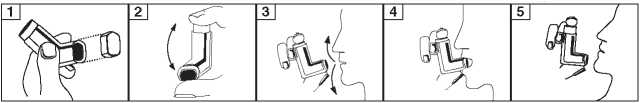



1. You should either stand up or sit upright when using the inhaler.
2. Remove the mouthpiece cover. Check the mouthpiece inside and outside to see that it is clean (figure 1).
3. Shake the inhaler well before use (figure 2) to ensure that any loose objects are removed and that the contents of the inhaler are evenly mixed.
4. Hold the inhaler upright with your thumb on the base, below the mouthpiece.
5. Breathe out as far as is comfortable (figure 3).
6. Place the mouthpiece in your mouth between your teeth and close your lips around it but do not bite it (figure 4).
7. Just after starting to breathe in as slowly as you can through your mouth, press down on top of the inhaler to release a puff into your mouth while still breathing in steadily and deeply (figure 4).
8. While holding your breath, take the inhaler from your mouth and take your finger from the top of the inhaler. Continue holding your breath for as long as is comfortable (figure 5).
9. If you are to take a second puff, wait about half a minute before repeating steps 3 to 8.
10. After use always replace the mouthpiece cover to keep out dust and fluff.The mouthpiece cover is replaced by firmly pushing and snapping it into position.
Practise in front of a mirror the first few times. If you see "mist" coming from the top of your inhaler or the sides of your mouth you should start again.
If you find it difficult to use the inhaler, a Volumatic® spacer device may help to overcome this problem. Contact your doctor, asthma nurse or pharmacist. If you do need to use the Volumatic® spacer device, please refer to the instruction leaflet provided with the spacer device, which includes all the information you need to use the spacer correctly.
If the inhaler gets very cold, take the metal canister out of the plastic case and warm it in your hands for a few minutes. Never use anything else to warm it up. After warming up the inhaler, spray one puff in the air before use.
Cleaning your inhaler
To prevent your inhaler from getting blocked up it should be cleaned at least once a week.
Cleaning your inhaler:
• Remove the mouthpiece cover.
• Do not remove the canister from the plastic casing at any time.
• Wipe the inside of the mouthpiece and the plastic casing with a dry cloth or tissue.
• Release one puff into the air before next using the inhaler to test the inhaler.
• Replace the mouthpiece cover.
Do not put the metal canister into water.
If you take more Vertine® Inhaler than you should
It is important to use the inhaler as instructed. If you accidentally take a larger dose than recommended you should contact your doctor, asthma nurse or pharmacist. You may notice your heart beating faster than usual and that you feel shaky and/or dizzy. You may also have a headache, muscle weakness and aching joints.
If you forget to use Vertine® Inhaler
If you forget to use your inhaler you should take the next dose when it is due. Do not take a double dose to make up for a dose you have forgotten.
Ifyou have any further questions on the use of this product, askyour doctor, asthma nurse or pharmacist.
POSSIBLE SIDE EFFECTS
Like all medicines, Vertine® Inhaler can cause side effects, although not everybody gets them. To reduce the chance of side effects your doctor will prescribe the lowest dose to control your asthma or COPD.The following side effects have been reported by patients using salmeterol.
Allergic reactions: you may notice that your breathing suddenly gets worse after using salmeterol. You may experience:
• Wheezing or coughing.
• Rash, itching, swelling (usually of the face, lips, tongue or throat).
Ifyou experience any of these side effects or if they occur suddenly after using salmeterol you should tell your doctor immediately. Allergic reactions to salmeterol are very rare (occurring in less than 1 in 10,000 patients).
Other side effects are:
Common (less than 1 in 10 patients, but more than 1 in 100)
• Muscle cramps.
• Feeling shaky, fast or irregular heartbeat (palpitations), headache, shaking hands (tremor). Tremors are more likely to occur ifyou are taking more than two puffs twice daily.These side effects do not last long and occur less as treatment with salmeterol continues.
Uncommon (less than 1 in 100 patients, but more than 1 in 1,000)
• Rash.
• Very fast heart rate (tachycardia).This is more likely to occur ifyou are taking more than two puffs twice daily.
• Feeling nervous.
Rare (less than 1 in 1,000 patients, but more than 1 in 10,000)
• Dizziness.
• Difficulty in sleeping (insomnia).
• A fall in the amount of potassium in the blood (you may notice an irregular heartbeat, muscle weakness and/or muscle cramps). Your doctor may ask you to have blood tests to check the amount of potassium in your blood from time to time. Ifyou have any concerns discuss them with your doctor or asthma nurse.
Very rare (less than 1 in 10,000 patients)
• Breathing difficulties or wheezing that gets worse straight after using Vertine® Inhaler. If this happens stop using your Vertine® Inhaler.
Use your fast-acting "reliever" inhaler to help your breathing and tell your doctor straight away.
• Irregular heartbeat or your heart gives an extra beat (arrhythmia). If this happens do not stop using Vertine® Inhaler but tell your doctor or asthma nurse.
• An increase in the amount of sugar (glucose) in your blood (hyperglycaemia). Ifyou have diabetes your doctor may want to monitor your blood sugar more frequently than usual and may need to adjust the treatment you take for your diabetes.
• Sore mouth or throat.
• Feeling sick (nausea).
• Aching, swollen joints.
• Chest pain.
If any of the side effects gets serious, or ifyou notice any side effects not listed
in this leaflet, please tell your doctor, asthma nurse or pharmacist.
HOWTO STORE VERTINE® INHALER
• Keep out of the reach and sight of children.
• Store below 30°C.
• Do not freeze.
• This canister contains a pressurised liquid. Do not expose to temperatures higher than 50°C.
• Do not puncture, break or burn the canister, even when apparently empty.
Do not use Vertine® Inhaler after the expiry date which is stated on the label and the carton after the letters EXP. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist howto dispose of medicines no longer required.These measures will help to protect the environment.
FURTHER INFORMATION
What Vertine® Inhaler contains
• The active substance is salmeterol (as xinafoate).
Each metered dose (ex-valve) contains 25 micrograms salmeterol (as xinafoate).This is equivalent to a delivered dose (ex-actuator) of 21 micrograms salmeterol (as xinafoate).
• The other ingredients are anhydrous ethanol, soya lecithin (E322) and norflurane (HFA 134a).
What Vertine® Inhaler looks like and contents of the pack
Vertine® Inhaler is a mid-green inhaler holding an aluminium canister with a pale-green mouthpiece cover. Each canister contains 120 actuations, each actuation containing 25 micrograms salmeterol (as xinafoate).
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne, BN22 9AG, United Kingdom.
Manufacturer
Fannin (UK) Limited, 42-46 Booth Drive, Park Farm South, Wellingborough, Northhamptonshire, NN8 6GT, UK.
This leaflet was last revised in 05/2013.
PL 00289/1667
50011-E
|
PHARMACODE |
V3idV | ||
|
AREA |
3QODVIAiyVHd | ||
|
-4 |
<-> |
► | |
|
30mm | |||