Vexol 1% (10Mg/Ml) Eye Drops Suspension
Package Leaflet - Information for the User VEXOL10 mg/ml eye drops, suspension Rimexolone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What VEXOL is and what it is used for
2. What you need to know before you use VEXOL
3. How to use VEXOL
4. Possible side effects
5. How to store VEXOL
6. Contents of the pack and other information
1 What VEXOL is and what it is used for
VEXOL belongs to a group of medicines known as corticosteroids.
It is used to prevent or treat eye inflammation following surgery of the eye and to treat inflammation of the eye surface and the front portion inside the eye (anterior segment).
It helps to relieve the symptoms of inflammation such as redness, soreness and swelling.
2 What you need to know before you use VEXOL Do not use VEXOL...
• If you have any type of infection of the eye that is not being treated. Use of steroids may make infections worse.
• If you have a red eye that has not been seen by a doctor.
• If you are allergic to rimexolone or any of the other ingredients of this medicine (listed in section 6).
Ask your doctor for advice.
VEXOL is not for use in CHILDREN.
Take special care...
• If you have a disorder causing a thinning of the eye tissues, such as rheumatoid arthritis, Fuch's dystrophy or following a corneal transplant. Steroids may cause further thinning and possible perforation.
• If you are diabetic, have a family history of glaucoma, or are very near - sighted please consult your doctor. The risk of developing an increase in eye pressure and/or cataract formation is higher in susceptible patients using steroids.
• Steroids applied to the eye may delay the healing of your eye wound Topical NSAIDs (non-steroidal anti inflammatory drug-medications- (type of painkillers)) are also known to slow or delay healing. Simultanous use of topical NSAIDs and topical steroids may increase the potential for healing problems.
You may still be able to use VEXOL, but discuss it with your doctor first.
Pregnancy and breast-feeding
If you are pregnant or might get pregnant, or if you are breast-feeding a baby, talk to your doctor before you use VEXOL.
Driving and using machines
If your vision is blurred or your sight is affected in any way following the use of VEXOL you should not drive or operate machinery until your vision is clear.
Other medicines and VEXOL
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Tell your doctor if you are using topical NSAIDs. Simultaneous use of topical steroids and topical NSAIDs may increase corneal healing problems.
If more than one topical ophthalmic medicinal product is being used, the medicines must be administered at least 5 minutes apart. Eye ointments should be administered last. Important information if you wear Contact Lenses
Wearing contact lenses is not advisable during treatment of an eye inflammation as it may make your condition worse.
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in VEXOL (benzalkonium chloride) that can discolour soft contact lenses and may cause eye irritation.
3 How to use VEXOL
The usual dose
This will depend on the reason for use.
Inflammation (red, painful eye)
Apply 1 drop in the affected eye 4 times a day or more often, if advised by your doctor.
Inflammation of the inside of the eye (Uveitis)
1st week - Apply 1 drop in the affected eye every hour while awake.
2nd week - Apply 1 drop every 2 hours while awake.
3rd week - Apply 1 drop 4 times a day.
4th week - Apply 1 drop twice daily for the first 4 days and then apply 1 drop once daily for the last 3 days.
Inflammation after eye surgery
Apply 1 drop in the affected eye 4 times a day, beginning 24 hours after your operation and continuing for the first 2 weeks afterwards.
Not for use in CHILDREN.
Remove the loose collar from the cap when the bottle is first opened.
Always use VEXOL exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
• Pull down your lower eyelid with a finger, until there is a 'pocket' between the eyelid and your eye. The drop will go in here (picture 1).
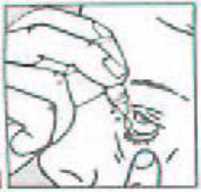
1
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops.
• Gently press on the base of the bottle to release one drop at a time (picture 2).
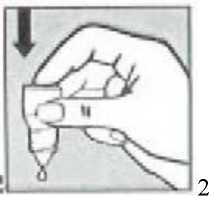
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• If a drop misses your eye, try again.
• If you forget to use VEXOL, just use it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your normal dosing schedule. Do not take a double dose to make up.
• If you use more VEXOL than you should, it can be washed out with luke warm water.
• If treatment with VEXOL is stopped...
A flare-up of inflammation may occur if treatment is discontinued early. Do not suddenly stop using the product without your doctor's advice. Your doctor may want to gradually reduce the amount you use to reduce the chance of unwanted effects.
If you have any further questions on the use of VEXOL, ask your doctor or pharmacist.
4 Possible side effects
Like all medicines, VEXOL can cause side effects, although not everybody gets them.
• You may experience some or all of the following effects in your eye(s):
Common (affect 1 to 10people in 100): Blurred vision, discharge, discomfort
Uncommon (affect 1 to 10people in 1000): Redness of the eye or inside the eyelid, pain, itching, dry eyes, swelling watery eyes, eye staining, , irritation, serious infections, , increased pressure in the eye, eye surface inflammation or other disorders.
Rare (1 to 10 users in 10,000): Swelling of the back of the eye, eyelid swelling, sensitivity to light, crusting on the eyelids or scarring or other disorders.
Not known (cannot be estimated from the available data): Reduced vision.
• You may also experience effects in other areas of your body including:
Uncommon (affect 1 to 10people in 1000): Headache, redness or soreness of the throat, bad or unusual taste.
Rare (1 to 10 users in 10,000): Allergy, low blood pressure, runny nose.
Not known (cannot be estimated from the available data): Chest pain.
• If VEXOL is used for a long time this can lead to an increase in pressure inside the eye or the formation of cataracts, both of which can lead to decreased vision. It can also lead to infections, as your natural resistance to these is reduced.
The risk of VEXOL induced increased pressure inside the eye may be greater in children and may occur earlier than in adults. VEXOL is not approved for use in children.
If any of the side effects get serious, or you notice any side effects not listed in this leaflet, tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
United Kingdom
Yellow card scheme
Website: www.mhra.gov.uk/yellowcard
5 How to store
• Keep out of the reach and sight of children.
• Do not freeze or store above 30°C.
• Stop using the bottle 4 weeks after first opening, to prevent infections.
• Do not use the drops after the expiry date (marked 'Exp') on the bottle and the carton. The expiry date refers to the last day of that month.
• Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• Do not pass this medicine on to others. It may harm them, even if their symptoms are the same as yours.
6 Contents of the pack and other information
What VEXOL contains
• The active substance is rimexolone. One ml of suspension contains 10 mg of rimexolone.
• The other ingredients are benzalkonium chloride, mannitol (E421), carbomer, polysorbate 80(E433), disodium edetate, sodium chloride, hydrochloric acid and/or sodium hydroxide (to adjust pH) and purified water.
What VEXOL looks like and contents of the pack
VEXOL is a white to off-white suspension supplied in a pack containing a 3 ml, 5 ml or
10 ml plastic bottle with a screw cap.
Marketing authorisation holder:
Alcon Laboratories (UK) Ltd.
Frimley Business Park
Frimley
Camberley
Surrey, GU16 7SR
United Kingdom
Manufacturer:
SA Alcon-Couvreur NV Rijksweg 14, B-2870 Puurs, Belgium.
Alcon Cusi S.A., Camil Fabra 58,
08320 El Masnou, Barcelona, Spain.
This medicinal product is authorized in the Member States of the EEA under the following names:
Austria, Finland, Germany, Greece, Ireland, Italy, Portugal, Spain, Sweden, UK: VEXOL This leaflet was last revised in May 2016
Alcon logo
7