Vividrin Eye Drops
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Vividrin Eye Drops Vividrin Hay Fever Eye Drops
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active
Sodium cromoglycate EP 2.00 %w/v
3 PHARMACEUTICAL FORM
Sterile Preserved Multidose Eye Drops
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Vividrin Eye Drops are for the prophylaxis and symptomatic treatment of acute allergic conjunctivitis, chronic allergic conjunctivitis and vernal kerato conjunctivitis.
Vividrin Hay Fever Eye Drops are for the treatment of acute seasonal allergic conjunctivitis.
4.2
Posology and method of administration
Route of Administration:
For topical administration to the eye.
Adults, Children and the Elderly
One or two drops into each eye up to four times daily.
4.3 Contraindications
Known hypersensitivity to benzalkonium chloride, sodium cromoglycate or other constituents.
4.4 Special warnings and precautions for use
The solution should be discarded 1 month after first opening the bottle or if any turbidity develops. Do not use if the bottle has been opened prior to receipt. Vividrin Eye Drops and Vividrin Hay Fever Eye Drops contain benzalkonium chloride. Do not wear soft contact lenses during the period of use.
4.5 Interaction with other medicinal products and other forms of interaction
None known.
4.6 Pregnancy and lactation
Use in Pregnancy: Vividrin Eye Drops and Vividrin Hay Fever Eye Drops should be used cautiously during pregnancy and lactation. The widespread use of sodium cromoglycate has yet to reveal any adverse effects to mother or child during pregnancy.
Use in Lactation: It is not known whether sodium cromoglycate is excreted in human breast milk, but on the basis of its physicochemical properties, this is considered unlikely. There is no information to suggest that the use of sodium cromoglycate has any undesirable effects on the baby.
4.7 Effects on ability to drive and use machines
Transient stinging or blurred vision may occur on instillation. Do not drive or operate machinery until proper vision is restored.
4.8 Undesirable effects
Transient stinging and burning may occur after instillation, other symptoms of local irritation have been reported rarely.
4.9 Overdose
As sodium cromoglycate is absorbed only to a very limited extent from eye drops, no action other than medical observation shouldbe necessary.
5 PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
5.1
Sodium cromoglycate has neither anti-histaminic or anti-inflammatory activity. Evidence suggests that sodium cromoglycate inhibits the release of mediators of the allergic reaction by stabilising the membranes of sensitised mast cells.
5.2 Pharmacokinetic properties
Due to lipid insolubility, sodium cromoglycate is poorly absorbed following administration to the eye. In normal volunteers approximately 0.03% is systemically absorbed. Absorbed sodium cromoglycate is excreted unchanged in the bile and urine.
Trace amounts of sodium cromoglycate have been detected in the aqueous humor of rabbits for up to 24 hours after treatment.
5.3 Preclinical safety data
The results of the studies do not add to the information needed by the prescriber, consequently, they are not repeated in the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Benzalkonium chloride EP Edetic acid disodium salt 2H20 EP Polysorbate 80 EP Sorbitol EP Sodium hydroxide BP Purified water EP
6.2 Incompatibilities
Sodium cromoglycate forms insoluble complexes with metal ions resulting in solution turbidity.
6.3 Shelf life
Unopened : 36 months
Once opened for the first time : 1 month
6.4 Special precautions for storage
Store below 25°C, out of direct sunlight
6.5 Nature and contents of container
Vividrin Eye Drops - 13.5ml polyethylene multidose eye dropper bottle with polyethylene cap. Vividrin Hay Fever Eye Drops - 10.0ml polyethylene multidose eye dropper bottle with polyethylene cap.
6.6 Special precautions for disposal
The following instructions for use are included in the patient leaflet.
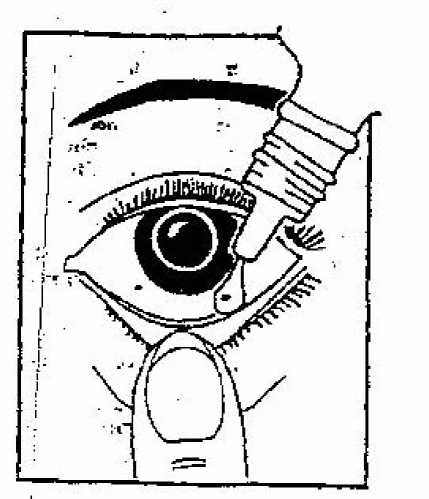
1. Before using your Vividrin Eye Drops (Vividrin Hay Fever Eye Drops) you should wash your hands.
2. You may find it helps to sit in front of a mirror, so that you can see what you are doing. Gently pull down your lower eyelid and carefully place one or two Vividrin Eye Drops (Vividrin Hay Fever Eye Drops) in the space between the eyelid and the eye. Take care not to touch the dropper with your eye or fingers.
3. Release the eyelid and blink a few times to make sure the liquid covers the whole surface of the eye.
4. Repeatthe procedure with the other eye and then replace the cap on the bottle.
7 MARKETING AUTHORISATION HOLDER
Bausch & Lomb (UK) Ltd
Bausch &Lomb House 106 London Road Kingston-Upon-Thames Surrey KT2 6TN
UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 03468/0035
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
13 December 1996
10
DATE OF REVISION OF THE TEXT
28/03/2011