Vividrin Nasal Spray
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Vividrin Nasal Spray
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active
Sodium cromoglycate EP 2.00% w/v
3 PHARMACEUTICAL FORM
Nasal spray
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For the prevention and relief of seasonal and perennial allergic rhinitis.
4.2 Posology and method of administration
Route of Administration For intra-nasal use.
Adults, Children and the Elderly
One spray into each nostril four to six times daily.
Note: Due to the prophylactic nature of sodium cromoglycate the patient should be encouraged to continue use even when symptoms have ceased.
4.3 Contraindications
Known hypersensitivity to benzalkonium chloride, sodium cromoglycate or other constituents.
4.4 Special warnings and precautions for use
Addition of Vividrin Nasal spray to existing sodium cromoglycate or steroid controlled asthma therapy may exaggerate the effects of that therapy.
4.5 Interaction with other medicinal products and other forms of interaction
It may be possible to reduce concomitant antihistamine therapy.
4.6 Fertility, pregnancy and lactation
Use in Pregnancy: Vividrin Nasal Spray should be used cautiously during pregnancy and lactation. The widespread use of sodium cromoglycate has yet to reveal any adverse effects to mother or child during pregnancy.
Use in Lactation: It is not known whether sodium cromoglycate is excreted in human breast milk but on the basis of its physicochemical properties, this is considered unlikely. There is no information to suggest that the use of sodium cromoglycate has any undesirable effects on the baby.
4.7 Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
Occasional irritation of the nasal mucosa may occur during the first days of use. In rare cases wheezing or tightness of the chest have been reported by patients.
4.9 Overdose
As sodium cromoglycate is absorbed only to a very limited extent in overdose, no action other than medical observation should be necessary.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Sodium cromoglycate has neither anti-histaminic or anti-inflammatory activity. Evidence suggests that sodium cromoglycate inhibits the release of mediators of the allergic reaction by stabilising the membranes of sensitised mast cells.
5.2 Pharmacokinetic properties
Sodium cromoglycate is poorly absorbed from the gastrointestinal tract. (Following inhalation as a fine powder only about 8% of a dose is reported to be deposited in the lungs from where it is rapidly absorbed and excreted unchanged in the urine and bile). Less than 7% of an intranasal dose is absorbed. The majority of an inhaled or an intranasal dose is swallowed, rapidly absorbed and excreted unchanged in the faeces.
5.3 Preclinical safety data
The results of the studies do not add to the information needed by the prescriber, consequently, they are not repeated in the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Benzalkonium Chloride Edetic Acid Disodium Salt 2H2O Polysorbate 80 Sorbitol
Sodium Hydroxide Purified Water
6.2 Incompatibilities
Sodium cromoglycate forms insoluble complexes with metal ions resulting in solution turbidity.
6.3 Shelf life
36 months.
6.4 Special precautions for storage
Store below 25°C, out of direct sunlight.
6.5 Nature and contents of container
15ml polyethylene bottles fitted with integral nasal spray pump and cap.
6.6 Special precautions for disposal
The following instructions for use are included in the patient leaflet for Vividrin Nasal Spray.
1. Blow your nose to clear any mucus. Take the cap off the spray and check that the nozzle is clean.
2. Hold the bottle with the spray nozzle pointing upwards, your fingers either side of the nozzle and your thumb on the base of the bottle. Press down with both fingers to ensure a fine spray is released.
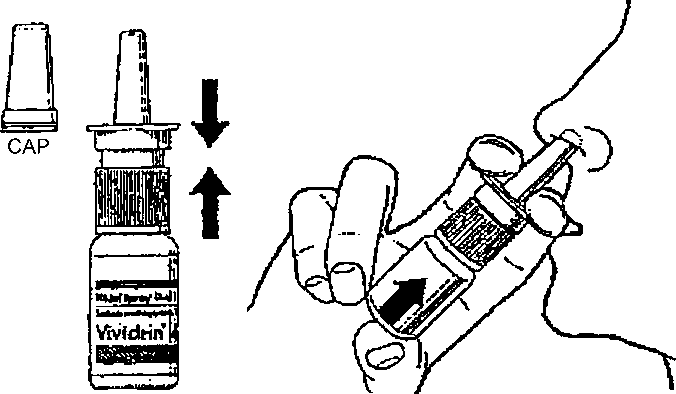
3. Holding the bottle upright, insert the spray nozzle into a nostril. Press the bottom of the bottle once with your thumb and a single spray of solution will be dispensed. You should then repeat the procedure for your other nostril.
4. After use wipe the nozzle with a clean tissue and replace the cap.
7 MARKETING AUTHORISATION HOLDER
Bausch & Lomb (UK) Ltd
Bausch & Lomb House
106 London Road
Kingston-Upon-Thames
Surrey
KT2 6TN
UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 03468/0034
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
13/12/1996 / 16/12/2008
10 DATE OF REVISION OF THE TEXT
28/03/2011