Vizioblok 2.5 Mg/Ml Eye Drops Solution
Package leaflet: Information for the user
Vizioblok 2.5 mg/ml Eye Drops, Solution
timolol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Vizioblok is and what it is used for
2. What you need to know before you use Vizioblok
3. How to use Vizioblok
4. Possible side effects
5. How to store Vizioblok
6. Contents of the pack and other information
1. What Vizioblok is and what it is used for
Vizioblok contains a substance called timolol which belongs to a group of medicines called beta-blockers. Timolol lowers the pressure in your eye(s). It is used to treat chronic open-angle and secondary glaucoma, when the pressure in the eye is raised, in adults.
Vizioblok eye drops solution is a sterile solution that does not contain a preservative.
2. What you need to know before you use Vizioblok
Do not use Vizioblok if:
• you are allergic to timolol, beta-blockers or any of the other ingredients of this medicine (listed in section 6);
• you have now or have had in the past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough);
• you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats);
• you have “cardiogenic shock” - a serious heart condition caused by very low blood pressure, which may result in the following symptoms: dizziness and lightheadedness, fast pulse rate, white skin, sweating, restlessness, loss of consciousness.
If you are not sure whether you should use Vizioblok talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before using Vizioblok if you have now or have had in the past:
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure;
• closed-angle glaucoma, a condition in which the pressure inside your eye becomes too high;
• low blood pressure;
• disturbances of heart rate such as slow heart beat;
• breathing problems, asthma or chronic obstructive pulmonary disease;
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome);
• diabetes as timolol may mask signs and symptoms of low blood sugar;
• overactivity of the thyroid gland as timolol may mask signs and symptoms.
• muscle weakness
• tumor of the adrenal gland (pheochromocytoma)
• blood and body fluid abnormality (metabolic acidosis)
• pain in the legs when walking due to poor circulation in the legs (claudication)
• reduced kidney function
• you wear soft contact lenses. Vizioblok has not been studied in patients wearing contact lenses. If you wear contact lenses, you should consult your doctor before using Vizioblok.
If you have a history of contact hypersensitivity to silver, you should not use this product.
Tell your doctor before you have an operation that you are using Vizioblok as timolol may change effects of some medicines used during anaesthesia.
If you suspect that Vizioblok is causing an allergic reaction or hypersensitivity (for example, skin rash, or redness and itching of the eye) contact your doctor immediately.
Children and adolescents
Vizioblok should generally be used with caution in young patients. In newborns, infants and younger children Vizioblok should be used with extreme caution. If coughing, wheezing, abnormal breathing or abnormal pauses in breathing (apnoea) occur, the use of the medicine should be stopped immediately. Contact your doctor as soon as possible. A portable apnoea monitor may also be helpful.
Other medicines and Vizioblok
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Vizioblok can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma.
Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes.
It is important to tell your doctor before using Vizioblok if you are taking one or more of the following medicines:
• eye drops containing adrenaline
• a calcium antagonist, such as nifedipine, verapamil or diltiazem, often used to treat high blood pressure, angina, an abnormal heartbeat or Raynaud’s syndrome;
• guanethidine, a medicine often used to treat high blood pressure
• parasympathomimetic medicine, which may have been prescribed to help you pass urine. Parasympathomimetics are also a particular type of medicine which is sometimes used to help restore normal movements through the bowel.
• digoxin, a medicine used to relieve heart failure or treat abnormal heartbeat;
• quinidine, used to treat heart conditions and some types of malaria;
• antidepressants known as fluoxetine and paroxetine;
• antiarrhythmics (including amiodarone);
• clonidine, a medicine used to treat high blood pressure;
• other beta-blockers taken by mouth or used as eye drops, because they belong to the same group of medicines as Vizioblok and could have an additive effect.
• insulin and other oral anti-diabetic drugs
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Use in pregnancy
Do not use Vizioblok if you are pregnant unless your doctor considers it necessary.
Use in breast-feeding
Do not use Vizioblok if you are breast-feeding. Timolol may get into your milk.
Driving and using machines
There are possible side effects associated with Vizioblok, such as dizziness, tiredness and changes in your eyesight, such as blurred vision, drooping of the upper eyelid (making the eye stay half closed), double vision which may affect your ability to drive and/or operate machinery. Do not drive and/or operate machinery until you feel well and your vision is clear.
3. How to use Vizioblok
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The doctor will decide how many drops you should take each day and how long you should use them.
The recommended dose is one drop in the affected eye(s) once or twice (one in the morning and one in the evening) each day.
Do not change your recommended dose without talking to your doctor.
Do not allow the tip of the multi dose container to touch the eye or areas around the eye. It could cause injury to your eye. The eye drops solution may become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision.
To avoid possible contamination of the multi-dose container, keep the tip of the multi-dose container away from contact with any surface.
Instructions for use
Before instillation of the eye drops:
- Wash your hands before opening the bottle.
- Do not use this medicine if you notice that the tamper-proof seal on the bottle neck is broken before you first use it.
- At the first use, after cap removal, without positioning the eyedropper above the eye, slowly squeeze bottle to deliver one drop in the air to get use to the pressure and time required to deliver one drop. In case of difficulties of delivering one drop at a time, this step might be repeated.
- Choose the position that you find most comfortable for the instillation of the drops (you can sit down, lie on your back, or stand in front of a mirror).
Instillation:
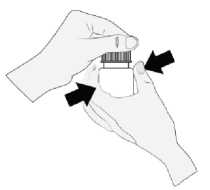
1) Hold the bottle directly below the cap and turn the cap to open the bottle. Do not touch
anything with the tip of the bottle to avoid contamination of the solution.
2) Tilt your head backwards and hold the bottle above your eye.
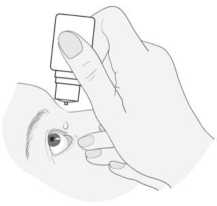
3) Pull the lower eyelid down and look up. Squeeze the bottle gently in the middle and let a drop fall into your eye. Please note that there might be a few seconds delay between squeezing and the drop coming out. Do not squeeze too hard.
If you are not sure how to administer your medicine, ask your doctor, pharmacist or nurse.
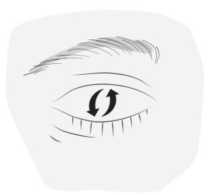
4) Blink a few times so that the drop spreads over the eye.
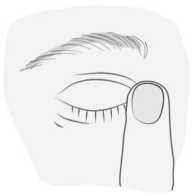
5) After using Vizioblok, press a finger into the corner of your eye, by the nose for 2 minutes. This helps to stop timolol getting into the rest of your body.
6) Follow the instructions 2. - 5. for dropping also into the other eye.
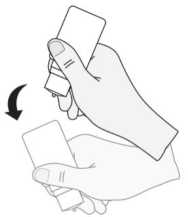
7) After use and prior to recapping, the bottle should be shaken once in a downwards direction, without touching the dropper tip, in order to remove any residual liquid on the tip. This is necessary in order to ensure delivery of subsequent drops.
There might remain a small amount of solution in the bottle at the end. This is accounted for by an overfill of all bottles ensuring that 5 ml solution can always be dispensed.
Do not use the eye drops for longer than 28 days after first opening the bottle.
Use in children and adolescents
Before your child starts to use Vizioblok, your child's doctor will have carried out a detailed medical examination and decided whether or not this medicine is suitable. Your child, especially a newborn, should be closely monitored for one to two hours after the first dose and carefully monitored for any signs of side effects.
Method of administration for use in children:
One drop only of Vizioblok should be instilled into the affected eye(s) each time. Follow the "Instructions for Use" above when administering the eye drops.
Duration of treatment for use in children
Your child's doctor will decide for how long the eye drops will be needed.
If you use more Vizioblok than you should
If you put too many drops in your eye or swallow any of the drops, you may:
• have a headache;
• feel dizzy or light-headed;
• have difficulty breathing;
• chest pain;
• feel that your heart rate has slowed down.
If this happens, contact your doctor immediately.
If you forget to use Vizioblok
It is important to take Vizioblok as prescribed by your doctor.
• If you miss a dose, use the drops as soon as possible.
• If it is almost time for the next dose, skip the missed dose and take the next dose at the usual time.
• Do not take a double dose to make up for the forgotten dose.
If you stop using Vizioblok
If you want to stop using this medicine talk to your doctor first. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Vizioblok without speaking to your doctor.
Serious side effects
See your doctor immediately if you notice any of the following side effects for which you
may need urgent medical treatment:
Rare (may affect up to 1 in 1000 people)
• Severe sudden life-threatening allergic reaction with sudden rash, swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty breathing or swallowing, and fainting (within minutes to hours) due to hypersensitivity (anaphylactic reaction)
• Paralysis, speech disorders, loss of consciousness due to reduced blood supply to the brain, interference with the blood supply to the brain which may lead to a stroke
• Changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure
• Difficulty breathing, shortness of breath, wheezing, cough
• Very slow pulse
• Sudden decreased vision after surgery for glaucoma
• Rash on the face, kidney inflammation, fever, joint and muscle pain due to connective tissue disease. A condition called lupus (systemic lupus erythematosus)
• Increases in signs and symptoms of myasthenia gravis (a muscle disorder which causes muscle weakness and fatigue of the eye muscles and/or of the muscles used in swallowing, chewing and breathing)
• Raynaud's phenomenon (White, "dead" fingers and toes)
• Limping and leg pain when walking because there is a reduced blood supply to your legs
• In men, a condition which affects your penis called Peyronie’s disease. The signs may be abnormal curve, pain or hardening of the tissue of your penis.
Other side effects
Common (may affect up to 1 in 10 people)
• Blurred vision, signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of the eyelid , inflammation of the surface of the eye, irritation or pain in the eye, dry eyes, decreased corneal (layer at the front of the eye) sensitivity, corneal erosion (damage to the front layer of the eyeball), feeling of a foreign object in the eye, secretion
• Headache, dizziness
Uncommon (may affect up to 1 in 100 people)
• Fainting, general weakness, fatigue
• Depression
• Abnormal vision
• Nausea, indigestion
Rare (may affect up to 1 in 1000 people)
• General allergic reactions including localized and generalized rash, itchiness
• Double vision, drooping of the upper eyelid (making the eye stay half closed) and separation of one of the layers within the eyeball after surgery to reduce the pressure in the eye
• Low blood pressure
• Chest pain, palpitations, oedema (fluid build up)
• Difficulty sleeping (insomnia), nightmares, memory loss
• Decreased sex drive
• Diarrhoea, dry mouth
• Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis
• Ringing sound in the ears
• Unusual sensations like tingling or pins and needles
• Cold hands and feet
Like other medicines applied into eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with intravenous and/or oral beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example taken by mouth or injected. The following additional side effects were seen within the class of beta-blockers when used for treating eye conditions:
• Low blood glucose levels
• Corneal erosion (damage to the front layer of the eyeball), tearing and redness in the eyes
• Heart failure
• Severe allergic reactions with swelling and difficulty breathing
• Skin rash and itching
• Taste disturbances, abdominal pain, vomiting
• Muscle pain not caused by exercise
• Sexual dysfunction
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any
possible side effects not listed in this leaflet. You can also report side effects directly via:
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland
HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: http://www.hpra.ie E-mail: medsafety@hpra.ie
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Vizioblok
Keep this medicine out of the sight and reach of children.
This medicinal product does not require any special storage conditions.
You can use Vizioblok for 28 days after first opening the bottle.
Do not use Vizioblok after the expiry date which is stated on the carton and the bottle after EXP. The expiry date refers to the last day of that month. Make sure the container is properly closed.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Vizioblok contains
- The active substance is timolol maleate.
Vizioblok is available in two strengths:
• Each ml of Vizioblok 2.5 mg/ml solution contains 3.417 mg of timolol maleate (equivalent to 2.5 mg of timolol).
• Each ml of Vizioblok 5 mg/ml contains 6.834 mg of timolol maleate (equivalent to 5 mg of timolol).
- The other ingredients are: sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, sodium hydroxide (for pH adjustment), water for injection.
What Vizioblok looks like and contents of the pack
Vizioblok 2.5 mg/ml eye drops solution, is presented as a clear, colorless aqueous solution in a white opaque 11 ml LDPE bottle and white Novelia nozzle (HDPE and silicone) with a white HDPE cap.
Pack sizes: 1 or 2 bottles in cardbox.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
PharmaSwiss Ceska republika s.r.o.
Jankovcova 1569/2, 170 00 Praha 7, Czech Republic
Manufacturer
Pharmathen S.A.,
6 Dervenakion 15351 Pallini Attiki Greece
And
EXCELVISION
27 st. La Lombardiere, Zl La Lombardiere,
ANNONAY 07100 France
Marketing Authorisation Number
Vizioblok 2.5 mg/ml Eye Drops, Solution: PL 33616/0020
This leaflet was last revised in: June 2016