Voltarol 140 Mg Medicated Plaster
155 mm
125 mm
◄-
A
300 mm
15 mm 270 mm 15 mm
—-Pharma code XXXX—

15 mm K—
PACKAGE LEAFLET: INFORMATION FOR THE USER



For use in adolescents from 16 years of age and adults. Active substance: diclofenac sodium
Read all of this leaflet carefully before you start using this medicinal product because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor
or pharmacist. This includes any possible side effects not listed in this leaflet.
- You must talk to a doctor if you do not feel better or if you feel worse after 7 days.
What is in this leaflet
1. What Voltarol Medicated plaster is and what it is used for
2. What you need to know before you use Voltarol Medicated plaster
3. How to use Voltarol Medicated plaster
4. Possible side effects
5. How to store Voltarol Medicated plaster
6. Contents of the pack and other information
1. WHAT VoLTARoL MEDicATED pLAsTER
is and what it is used for
Voltarol Medicated plaster is a medicine that relieves pain.
It belongs to a group of medicines known as non-steroidal anti-inflammatory drugs (NSAIDs).
Voltarol Medicated plaster is used for the local symptomatic and short term treatment of pain associated with acute strains, sprains or bruises on the arms and legs as a result of injuries, e.g. sports injuries in adolescents from 16 years of age and adults.
You must talk to a doctor if you do not feel better or if you feel worse after 7 days.
IMPORTANT precautions
• If symptoms persist for longer than 7 days, you should see a doctor.
• The medicated plaster must not come into contact with or be applied to the eyes or mucous membranes.
• Elderly patients should use Voltarol Medicated plaster with caution because they are more likely to experience side effects.
After taking off the medicated plaster, avoid exposing the treated area to direct sunlight or solarium radiation in order to reduce the risk of sensitivity to light.
children and adolescents
Voltarol Medicated plaster should not be used in children and adolescents under 16 years of age because no adequate experience is available for this age group.
other medicines and Voltarol Medicated plaster
Please tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Do not use Voltarol Medicated plaster at the same time as any other diclofenac-containing or other non-steroidal pain-relieving and anti-inflammatory medicines regardless of whether these are used externally or taken by mouth.
Provided that Voltarol Medicated plaster is used
correctly, only a small amount of diclofenac is absorbed
into the blood. Therefore interactions with other
medicines seen with diclofenac products
taken by mouth, are unlikely.
pregnancy and breast-feeding
pregnancy
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
In the first 6 months of pregnancy or if you want to become pregnant, Voltarol Medicated plaster should be used only after talking to your doctor.
In the last 3 months of pregnancy, Voltarol Medicated plaster must not be used because an increased risk of complications for the mother and the child cannot be ruled out (see ”Do not use Voltarol Medicated plaster”).

39 mm 39 mm
-►-<-
Pharma code XXX
Y
2. what you need to know before you use voltarol medicated plaster
Do not use Voltarol Medicated plaster if you
- are allergic to diclofenac or any of the other ingredients in this medicine (listed in section 6);
- are allergic to any other non-steroidal antiinflammatory drug (NSAID, e.g. acetylsalicylic acid or ibuprofen);
- have ever developed asthma attacks, hives or swelling and irritation inside the nose after taking acetylsalicylic acid or any other NSAID;
- are suffering from an active stomach or intestinal ulcer;
- are in the last three months of pregnancy.
Voltarol Medicated plaster should not be used on injured skin (e.g. skin abrasions, cuts, burns), infected skin or skin affected by exudative dermatitis or eczema;
Warnings and precautions
Talk to your doctor or pharmacist before using Voltarol Medicated plaster
• if you suffer from disorders of the kidneys, heart or liver, or if you suffer or have previously suffered from a stomach or intestinal ulcer or intestinal inflammation or a tendency to bleeding.
Consult a doctor or pharmacist before using Voltarol Medicated plaster if any of the above mentioned applies to you.
Take special care with Voltarol Medicated plaster
• if you notice a skin rash. If this happens, immediately remove the medicated plaster and stop the treatment.
Side effects can be reduced by using the lowest effective dose for the shortest possible period of time.
Breast-feeding
Small quantities of diclofenac pass into the breast milk.
Talk to your doctor before using Voltarol Medicated plaster during breast-feeding. In any case, if you are breast-feeding Voltarol Medicated plaster should not be applied directly onto the breast area.
Driving and using machines
Voltarol Medicated plaster has no influence on your ability
to drive and use machines.
3. how to use voltarol medicated plaster
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is one medicated plaster twice daily.
Attach one medicated plaster to the painful area twice daily, in the morning and in the evening. The maximum total daily dose is 2 medicated plasters, even if there is more than one injured area to be treated. Treat only one painful area at a time.
use in children and adolescents
Voltarol Medicated plaster is not recommended for use in children and adolescents under 16 years of age. There are insufficient data of efficacy and safety available for children and adolescents below 16 years (see section 2).
in adolescents aged 16 years and over, if this product is required for more than 7 days for pain relief or if the symptoms worsen, please consult a doctor.
Method of administration
For treatment on the skin (cutaneous) use only.
Do not take by mouth.
►
■<
15 mm
155 mm
A A
LO
V
A
V
A
1. Cut the sachet along the dotted line and remove the medicated plaster
To apply the plaster:
2. Remove one of the two protective films.
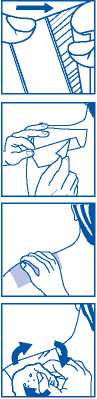
3. Apply to the area to be treated and remove the remaining protective film.
4. Apply slight pressure with the palms of your hand until complete adhesion to the skin is achieved.
5. Moisten the plaster with water and peel away an edge of the plaster and pull smoothly away from the skin.
6. To remove any product residues, wash the affected area with water gently rubbing the area with your fingers using a circular movement.
If necessary, the medicated plaster can be held in place using a net bandage.
Use the medicated plaster only on intact non-diseased skin.
Do not use the medicated plaster together with an air-tight (occlusive) bandage.
Do not wear it when bathing or showering.
Do not divide the medicated plaster, by cutting with scissors, for example.
Do not use Voltarol Medicated plaster for longer than 7 days. The use of this medicine for a longer period of time needs advice and must be discussed with a doctor.
If you have the impression that the effect of Voltarol Medicated plaster is too strong or too weak, please talk to your doctor or pharmacist.
If you apply more Voltarol Medicated plaster than you should
Please tell your doctor if you experience side effects after incorrect use of this medicine, if you apply more patches than you should or if a patch is accidently applied to a child. They will be able to advise you of any action that may need to be taken.
If you forget to use Voltarol Medicated plaster
You should apply a new patch to the affected area when you remember. Do not apply more than one patch to make up for the missed patch.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately and stop using the plaster if you notice any of the following:
sudden itchy rash (hives); swelling of the hands, feet, ankles, face, lips, mouth or throat; difficulty breathing; drop in blood pressure or weakness.
You may experience the following side effects:
Common side effects (may affect up to 1 in 10 people):
local skin reactions, such as skin redness, burning sensation, itching, inflamed skin redness, skin rash, sometimes with pustules or wheals.
Very rare side effects: may affect up to 1 in 10,000 people:
Hypersensitivity reactions or local allergic reactions (contact dermatitis).
In patients externally using drugs from the same drug group as diclofenac, there have been isolated reports of generalised skin rash, hypersensitivity reactions such as swelling of the skin and mucous membranes (such as lips, mouth and throat) and anaphylactic-type (severe allergic) reactions. Including problems with blood circulation and light sensitivity reactions.
Absorption of diclofenac into the body by the skin is very low compared to the drug concentration in the blood following diclofenac taken by mouth. Therefore, the likelihood of side effects occurring in the body as a whole (such as stomach or kidney problems or difficulty breathing) is very low.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. how to store voltarol medicated plaster
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton and the sachet after ”EXP”. The expiry date refers to the last day of that month.
Store below 30 °C.
Store in the original package in order to protect from desiccation and light.
Keep the sachet tightly closed in order to protect from desiccation and light.
Do not use Voltarol Medicated plaster if you notice that it is damaged.
Used plasters should be folded in half with the sticky side inwards.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. contents of the pack and other information
What Voltarol Medicated plaster contains
The active substance is diclofenac sodium.
Each medicated plaster contains 140 mg diclofenac sodium. The other ingredients are:
Supporting layer:
Polyester non-woven fabric Adhesive layer:
Basic butylated methacrylate coplymer Copolymer acrylate vinyl acetate PEG 12stearate Sorbitan oleate
Liner:
Mono silicone coated paper
What Voltarol Medicated plaster looks like and contents of the pack
Voltarol Medicated plaster are white 10x14 cm sized selfadhesive plasters made of non-woven fabric on one and paper on other side.
Voltarol Medicated plaster is available in packs of 2, 5 and 10 plasters, each in a single sachet.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Novartis Consumer Health UK Limited Park view, Riverside Way Watchmoor Park, Camberley Surrey GU15 3YL, United Kingdom
Manufacturer:
Novartis Consumer Health GmbH
Zielstattstr. 40
81379 Munich, Germany
SPA Italiana Laboratori Bouty
Strada Statale n. 11 Padana Superiore, km 160
20060 Cassina de' Pecchi (MI), Italia
This medicinal product is authorised in the Member states of the EEA under the following names:
DE: Diclofenac-Natrium Sophena 140 mg Wirkstoffhaltiges Pflaster
BE: Sophenoderm 140 mg pleister CZ: Voltaren 140 mg leciva naplast DK: Diclofenac Sophena EE: Voltaren EL: Voltadol
FI: Diclo sodium 140 mg laakelaastari
HU: Voltaren ActiGo140 mg gyogyszeres tapasz
LT: Voltaren 140 mg vaistinis pleistras
LV: Voltaren 140 mg arstnieciskais plaksteris
NO: Voltarol Medicated plaster 140 mg medisinert plaster
PT: Voltaren Plast 140 mg emplastro medicamentoso
SE: Voltaren 140 mg medicinskt plaster
SK: Voltaren 140 mg lieciva naplast'
UK: Voltarol Medicated plaster 140 mg medicated plaster
This leaflet was last approved in April 2015
GB 932727 & NOVARTIS
LO