Voltarol 50Mg Suppositories
S408 LEAFLET Voltarol 20140321
PACKAGE LEAFLET: INFORMATION FOR THE USER VOLTAROL 50mg SUPPOSITORIES (diclofenac sodium)
Your medicine is known as Voltarol 50mg suppositories but will be referred to as Voltarol Suppositories throughout the following patient information leaflet.
Information for other strengths of Voltarol Suppositories i.e. 12.5mg, 25mg and 100mg also may be present in this leaflet.
What you need to know about Voltarol Suppositories
Your doctor has decided that you need this medicine to help treat your condition.
Please read this leaflet carefully before you start to use these suppositories. It contains important information. Keep the leaflet in a safe place because you may want to read it again.
If you have any other questions, or if there is something you don't understand, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Voltarol Suppositories are, and what they are used for
2. Things to consider before you start to use Voltarol Suppositories
3. How to use Voltarol Suppositories
4. Possible side effects
5. How to store Voltarol Suppositories
6. Further information
1. WHAT VOLTAROL SUPPOSITORIES ARE, AND WHAT THEY ARE USED FOR
Diclofenac sodium, the active ingredient in Voltarol Suppositories, is one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs reduce pain and inflammation.
• Voltarol Suppositories relieve pain, reduce swelling and ease inflammation in conditions affecting the joints, muscles and tendons including:
- Rheumatoid arthritis, osteoarthritis, acute gout, ankylosing spondylitis
- Backache, sprains and strains, soft tissue sports injuries, frozen shoulder, dislocations and fractures
- Tendonitis, tenosynovitis, bursitis.
• They are also used to treat pain and inflammation associated with dental and minor surgery.
• In children aged 1 to 12 Voltarol Suppositories 12.5 and 25 mg are used to treat juvenile chronic arthritis.
• In children aged over 6 they can also be used alone, or in combination with other painkillers, for the short term treatment of any pain experienced after an operation.
2. THINGS TO CONSIDER BEFORE YOU START TO USE VOLTAROL SUPPOSITORIES
Some people MUST NOT use Voltarol Suppositories. Talk to
your doctor if:
• you think you may be allergic to diclofenac sodium, aspirin, ibuprofen or any other NSAID, or to any of the other ingredients of Voltarol Suppositories. (These are listed at the end of the leaflet.) Signs of a hypersensitivity reaction include swelling of the face and mouth (angioedema), breathing problems, runny nose, skin rash or any other allergic type reaction
• you have now, or have ever had, a stomach (gastric) or duodenal (peptic) ulcer, or bleeding in the digestive tract (this can include blood in vomit, bleeding when emptying bowels, fresh blood in faeces or black, tarry faeces)
• you have had stomach or bowel problems after you have taken other NSAIDs
• you have severe heart, kidney or liver failure
• if you have established heart disease and/or cerebrovascular disease e.g. if you have had a heart attack, stroke, mini-stroke (TIA) or blockages to blood vessels to the heart or brain or an operation to clear or bypass blockages
• if you have or have had problems with your blood circulation (peripheral arterial disease)
• you are more than six months pregnant
• you suffer from ineffectual straining to empty the bowels, diarrhoea or rectal bleeding
You should also ask yourself these questions before using
Voltarol Suppositories:
• Do you suffer from any stomach or bowel disorders including ulcerative colitis or Crohn's disease?
• Do you have kidney or liver problems, or are you elderly?
• Do you have a condition called porphyria?
• Do you suffer from any blood or bleeding disorder? If you do, your doctor may ask you to go for regular check-ups while you are using these suppositories.
• Have you ever had asthma?
• Are you breast-feeding?
• Do you have angina, blood clots, high blood pressure, raised cholesterol or raised triglycerides
• Do you have heart problems, or have you had a stroke, or do you think you might be at risk of these conditions (for example, if you have high blood pressure, diabetes or high cholesterol or are a smoker)?
• Do you have diabetes
• Do you smoke
• Do you have Lupus (SLE) or any similar condition?
If the answer to any of these questions is YES, discuss your treatment with your doctor or pharmacist because Voltarol Suppositories might not be the right medicine for you.
Are you taking other medicines?
Some medicines can interfere with your treatment. Tell your doctor or pharmacist if you are taking any of the following:
• Medicines to treat diabetes
• Anticoagulants (blood thinning tablets like warfarin)
• Diuretics (water tablets)
• Lithium (used to treat some mental problems)
• Methotrexate (for some inflammatory diseases and some cancers)
• Ciclosporin and tacrolimus (used to treat some inflammatory diseases and after transplants)
• Trimethoprim (a medicine used to prevent or treat urinary tract infections)
• Quinolone antibiotics (for infections)
• Any other NSAID or COX-2 (cyclo-oxgenase-2) inhibitor, for example aspirin or ibuprofen
• Mifepristone (a medicine used to terminate pregnancy)
• Cardiac glycosides (for example digoxin), used to treat heart problems
• Medicines known as SSRIs used to treat depression
• Oral steroids (an anti-inflammatory drug)
• Medicines used to treat heart conditions or high blood pressure, for example beta-blockers or ACE inhibitors.
• Voriconazole (a medicine used to treat fungal infections).
• Phenytoin (a medicine used to treat seizures)
• Colestipol/cholestyramine (used to lower cholesterol)
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Pregnancy
• Are you pregnant or planning to become pregnant? Although not common, abnormalities have been reported in babies whose mothers have taken NSAIDs during pregnancy. You should not use Voltarol Suppositories during the last 3 months of pregnancy as it may affect the baby's circulation.
• Are you trying for a baby? Using Voltarol Suppositories may make it more difficult to conceive. You should talk to your doctor if you are planning to become pregnant, or if you have problems getting pregnant.
Will there be any problems with driving or using machinery?
Very occasionally people have reported that Voltarol Suppositories have made them feel dizzy, tired or sleepy. Problems with eyesight have also been reported. If you are affected in this way, you should not drive or operate machinery.
Other special warnings
• You should take the lowest dose of Voltarol for the shortest possible time, particularly if you are underweight or elderly.
• There is a small increased risk of heart attack or stroke when you are taking any medicine like Voltarol. The risk is higher if you are taking high doses for a long time. Always follow the doctor's instructions on how much to take and how long to take it for.
• Whilst you are taking these medicines your doctor may want to give you a check-up from time to time.
• If you have a history of stomach problems when you are taking NSAIDs, particularly if you are elderly, you must tell your doctor straight away if you notice any unusual symptoms.
• Because it is an anti-inflammatory medicine, Voltarol may reduce the symptoms of infection, for example, headache and high temperature. If you feel unwell and need to see a doctor, remember to tell him or her that you are taking Voltarol.
• VOLTAROL Suppositories 50 mg and 100 mg are not suitable for children.
• VOLTAROL Suppositories 12.5 mg are not used for adults.
3. HOW TO USE VOLTAROL SUPPOSITORIES
The doctor will tell you how to use Voltarol Suppositories. Always follow his/her instructions carefully. The dose will be on the pharmacist's label. Check the label carefully. If you are not sure, ask your doctor or pharmacist. Keep using the suppositories for as long as you have been told, unless you have any problems. In that case, check with your doctor.
Suppositories are designed for insertion into the back passage (rectum). Never take them by mouth.
The doctor may also prescribe another drug to protect the stomach to be taken at the same time, particularly if you have had stomach problems before, or if you are elderly, or taking certain other drugs as well.
Adults
Voltarol Suppositories are normally inserted one, two or three times a day up to a maximum total daily dose of 150mg. The number of suppositories you need will depend on the strength which the doctor has given you.
Elderly
Your doctor may advise you to take a dose that is lower than the usual adult dose if you are elderly. Your doctor may also want to check closely that the Voltarol Suppositories are not affecting your stomach, particularly during the first 4 weeks that you are using the suppositories.
For the treatment of chronic juvenile arthritis in children aged 1 to 12:
Doses vary with age, but are usually between 1 and 3 mg/kg body weight every day divided into 2 or 3 doses.
For the treatment of post-operative pain in children aged 6 and over:
Doses vary with age, but are usually between 1 and 2 mg/kg body weight per day divided into 2 or 3 doses for no more than 4 days. Your child's doctor will work out the dose that is suitable for your child and will tell you how many Voltarol Suppositories to use and how often. Follow his/her instructions carefully. If you are not sure about the dose, check with your doctor or pharmacist.
How to insert the suppositories
• Empty your bowels before inserting a suppository.
• Wash your hands.
• Take out the strip of suppositories and tear off one along the perforation.
• Then take the suppository out of the plastic wrapping by pulling back the loose end.
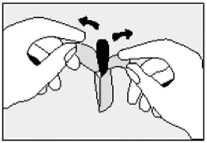
• Lie on one side with your knees pulled up towards your chest.
• Gently push the suppository pointed end first into your back passage (rectum) with your finger. Push the suppository in as far as possible as shown in the diagram.
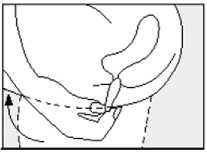
• Lower your legs and, if possible, stay still for a few minutes.
• If you feel as if you need to push the suppository out, try to resist this by lying still with your buttocks pressed together. It is important to keep the suppository in the rectum to allow it to melt and the medicine to be absorbed. Pushing the suppository high into the rectum with your finger will help to reduce this feeling.
• Wash your hands.
The procedure is the same for a child. Once they have emptied their bowels, get them to lie down on their front or side. Gently push the suppository into the child's back passage until it disappears. Try and stop the child moving around for a few minutes to reduce the risk of the suppository coming out.
What if you forget to take a dose?
If you forget to use a suppository, do not worry. Use one as soon as you remember. If it is nearly time for your next dose though, just take the next dose and forget about the one you missed. Do not double up the next dose to make up for the one you missed. Do not insert 2 suppositories at the same time. The total dose should not be more than 150 mg each day if you are an adult. Children should not take more than the dose that is prescribed by their doctor.
What if you use too many suppositories?
You should not take more than 150 mg in one day if you are an adult. Children should not take more than the dose that is prescribed by their doctor. If you accidentally use too many suppositories or use them too often, tell your doctor or go to your nearest casualty department straight away.
4. POSSIBLE SIDE EFFECTS
Voltarol Suppositories are suitable for most people, but, like all medicines, they can sometimes cause side effects. Side effects may be minimised by using the lowest effective dose for the shortest duration necessary.
Some side effects can be serious
Stop using the suppositories and tell your doctor straight away if you notice:
• Stomach pain, indigestion, heartburn, wind, nausea (feeling sick) or vomiting (being sick)
• Any sign of bleeding in the stomach or intestine, for example, when emptying your bowels, blood in vomit or black, tarry faeces
• Allergic reactions which can include skin rash, itching, bruising, painful red areas, peeling or blistering
• Wheezing or shortness of breath (bronchospasm)
• Swollen face, lips, hands or fingers
• Yellowing of your skin or the whites of your eyes
• Persistent sore throat or high temperature
• An unexpected change in the amount of urine produced and/or its appearance.
If you notice that you are bruising more easily than usual or have frequent sore throats or infections, tell your doctor.
Voltarol Suppositories may also occasionally cause itching or burning in your back passage or make any haemorrhoids (piles) worse.
The side effects listed below have also been reported.
Common side effects (These may affect between 1 and 10 in every 100 patients):
• Stomach pain, heartburn, nausea, vomiting, diarrhoea, indigestion, wind, loss of appetite
• Headache, dizziness, vertigo
• Skin rash or spots
• Raised levels of liver enzymes in the blood
• Irritation where the suppository is inserted.
• Stomach ulcers or bleeding (there have been very rare reported cases resulting in death, particularly in the elderly)
• Gastritis (inflammation, irritation or swelling of the stomach lining)
• Vomiting blood
• Diarrhoea with blood in it or bleeding from the back passage
• Black, tarry faeces or stools
• Drowsiness, tiredness
• Hypotension (low blood pressure, symptoms of which may include faintness, giddiness or light headedness)
• Skin rash and itching
• Fluid retention, symptoms of which include swollen ankles
• Liver function disorders, including hepatitis and jaundice Very rare side effects (These may affect less than 1 in every 10,000 patients):
Effects on the nervous system:
Tingling or numbness in the fingers, tremor, visual disturbances such as blurred or double vision, hearing loss or impairment, tinnitus (ringing in the ears), sleeplessness, nightmares, mood changes, depression, anxiety, mental disorders, disorientation and loss of memory, fits, headaches together with a dislike of bright lights, fever and a stiff neck, disturbances in sensation.
Effects on the stomach and digestive system:
Constipation, inflammation of the tongue, mouth ulcers, inflammation of the inside of the mouth or lips, taste changes, lower gut disorders (including inflammation of the colon, or worsening of ulcerative colitis or Crohn's disease).
Effects on the heart, chest or blood:
Palpitations (fast or irregular heart beat), chest pain, hypertension (high blood pressure), inflammation of blood vessels (vasculitis), inflammation of the lung (pneumonitis), heart disorders, including congestive heart failure or heart attack, blood disorders (including anaemia).
Effects on the liver or kidneys:
Kidney or severe liver disorders including liver failure, presence of blood or protein in the urine.
Effects on skin or hair:
Serious skin rashes including Stevens-Johnson syndrome, Lyell's syndrome and other skin rashes which may be made worse by exposure to sunlight.
Hair loss.
Other side effects that have also been reported include:
Inflammation of the pancreas, impotence. Facial swelling, inflammation of the lining of the brain (meningitis), stroke, throat disorders, confusion, hallucinations, malaise (general feeling of discomfort), inflammation of the nerves in the eye.
Do not be alarmed by this list - most people use Voltarol Suppositories without any problems.
If any of the symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor. He/she may want to give you a different medicine.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE VOLTAROL SUPPOSITORIES
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Voltarol Suppositories should not be stored above 30°C. Protect from heat.
• Do not use after the expiry date printed on the carton label or blister strip.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Voltarol Suppositories contain
• Each suppository contains 50mg of the active ingredient diclofenac sodium.
• Voltarol suppositories also contain the following inactive ingredients: hard fat.
What Voltarol Suppositories look like and contents of the pack
Voltarol suppositories are white to yellowish, torpedo-shaped, with smooth surfaces and a slightly fatty odour.
Voltarol suppositories are available as packs of 10.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1Dx.
Manufacturer
This product is manufactured by
• Famar ABE, Peristeriou, Greece.
• Famar ABE Factory 49 km Athens-Lamia 190 11 Avlona, Greece.
| POM | PL No: 19488/0408 Leaflet revision date: 21 March 2014
Voltarol is a registered trade mark of Novartis AG, Switzerland.
S408 LEAFLET Voltarol 20140321
S408 LEAFLET Diclofenac 20140321
PACKAGE LEAFLET: INFORMATION FOR THE USER DICLOFENAC SODIUM 50mg SUPPOSITORIES (diclofenac sodium)
Your medicine is known as Diclofenac sodium 50mg suppositories but will be referred to as Diclofenac Suppositories throughout the following patient information leaflet.
Information for other strengths of Diclofenac Suppositories i.e. 12.5mg, 25mg and 100mg also may be present in this leaflet.
What you need to know about Diclofenac Suppositories
Your doctor has decided that you need this medicine to help treat your condition.
Please read this leaflet carefully before you start to use these suppositories. It contains important information. Keep the leaflet in a safe place because you may want to read it again.
If you have any other questions, or if there is something you don't understand, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Diclofenac Suppositories are, and what they are used for
2. Things to consider before you start to use Diclofenac Suppositories
3. How to use Diclofenac Suppositories
4. Possible side effects
5. How to store Diclofenac Suppositories
6. Further information
1. WHAT DICLOFENAC SUPPOSITORIES ARE, AND WHAT THEY ARE USED FOR
Diclofenac sodium, the active ingredient in Diclofenac Suppositories, is one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs reduce pain and inflammation.
• Diclofenac Suppositories relieve pain, reduce swelling and ease inflammation in conditions affecting the joints, muscles and tendons including:
- Rheumatoid arthritis, osteoarthritis, acute gout, ankylosing spondylitis
- Backache, sprains and strains, soft tissue sports injuries, frozen shoulder, dislocations and fractures
- Tendonitis, tenosynovitis, bursitis.
• They are also used to treat pain and inflammation associated with dental and minor surgery.
• In children aged 1 to 12 Diclofenac Suppositories 12.5 and 25 mg are used to treat juvenile chronic arthritis.
• In children aged over 6 they can also be used alone, or in combination with other painkillers, for the short term treatment of any pain experienced after an operation.
2. THINGS TO CONSIDER BEFORE YOU START TO USE DICLOFENAC SUPPOSITORIES
Some people MUST NOT use Diclofenac Suppositories. Talk to
your doctor if:
• you think you may be allergic to diclofenac sodium, aspirin, ibuprofen or any other NSAID, or to any of the other ingredients of Diclofenac Suppositories. (These are listed at the end of the leaflet.) Signs of a hypersensitivity reaction include swelling of the face and mouth (angioedema), breathing problems, runny nose, skin rash or any other allergic type reaction
• you have now, or have ever had, a stomach (gastric) or duodenal (peptic) ulcer, or bleeding in the digestive tract (this can include blood in vomit, bleeding when emptying bowels, fresh blood in faeces or black, tarry faeces)
• you have had stomach or bowel problems after you have taken other NSAIDs
• you have severe heart, kidney or liver failure
• if you have established heart disease and/or cerebrovascular disease e.g. if you have had a heart attack, stroke, mini-stroke (TIA) or blockages to blood vessels to the heart or brain or an operation to clear or bypass blockages
• if you have or have had problems with your blood circulation (peripheral arterial disease)
• you are more than six months pregnant
• you suffer from ineffectual straining to empty the bowels, diarrhoea or rectal bleeding
You should also ask yourself these questions before using
Diclofenac Suppositories:
• Do you suffer from any stomach or bowel disorders including ulcerative colitis or Crohn's disease?
• Do you have kidney or liver problems, or are you elderly?
• Do you have a condition called porphyria?
• Do you suffer from any blood or bleeding disorder? If you do, your doctor may ask you to go for regular check-ups while you are using these suppositories.
• Have you ever had asthma?
• Are you breast-feeding?
• Do you have angina, blood clots, high blood pressure, raised cholesterol or raised triglycerides
• Do you have heart problems, or have you had a stroke, or do you think you might be at risk of these conditions (for example, if you have high blood pressure, diabetes or high cholesterol or are a smoker)?
• Do you have diabetes
• Do you smoke
• Do you have Lupus (SLE) or any similar condition?
If the answer to any of these questions is YES, discuss your treatment with your doctor or pharmacist because Diclofenac Suppositories might not be the right medicine for you.
Are you taking other medicines?
Some medicines can interfere with your treatment. Tell your doctor or pharmacist if you are taking any of the following:
• Medicines to treat diabetes
• Anticoagulants (blood thinning tablets like warfarin)
• Diuretics (water tablets)
• Lithium (used to treat some mental problems)
• Methotrexate (for some inflammatory diseases and some cancers)
• Ciclosporin and tacrolimus (used to treat some inflammatory diseases and after transplants)
• Trimethoprim (a medicine used to prevent or treat urinary tract infections)
• Quinolone antibiotics (for infections)
• Any other NSAID or COX-2 (cyclo-oxgenase-2) inhibitor, for example aspirin or ibuprofen
• Mifepristone (a medicine used to terminate pregnancy)
• Cardiac glycosides (for example digoxin), used to treat heart problems
• Medicines known as SSRIs used to treat depression
• Oral steroids (an anti-inflammatory drug)
• Medicines used to treat heart conditions or high blood pressure, for example beta-blockers or ACE inhibitors.
• Voriconazole (a medicine used to treat fungal infections).
• Phenytoin (a medicine used to treat seizures)
• Colestipol/cholestyramine (used to lower cholesterol)
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Pregnancy
• Are you pregnant or planning to become pregnant? Although not common, abnormalities have been reported in babies whose mothers have taken NSAIDs during pregnancy. You should not use Diclofenac Suppositories during the last 3 months of pregnancy as it may affect the baby's circulation.
• Are you trying for a baby? Using Diclofenac Suppositories may make it more difficult to conceive. You should talk to your doctor if you are planning to become pregnant, or if you have problems getting pregnant.
Will there be any problems with driving or using machinery?
Very occasionally people have reported that Diclofenac Suppositories have made them feel dizzy, tired or sleepy. Problems with eyesight have also been reported. If you are affected in this way, you should not drive or operate machinery.
Other special warnings
• You should take the lowest dose of Diclofenac for the shortest possible time, particularly if you are underweight or elderly.
• There is a small increased risk of heart attack or stroke when you are taking any medicine like Diclofenac. The risk is higher if you are taking high doses for a long time. Always follow the doctor's instructions on how much to take and how long to take it for.
• Whilst you are taking these medicines your doctor may want to give you a check-up from time to time.
• If you have a history of stomach problems when you are taking NSAIDs, particularly if you are elderly, you must tell your doctor straight away if you notice any unusual symptoms.
• Because it is an anti-inflammatory medicine, Diclofenac may reduce the symptoms of infection, for example, headache and high temperature. If you feel unwell and need to see a doctor, remember to tell him or her that you are taking Diclofenac.
• DICLOFENAC Suppositories 50 mg and 100 mg are not suitable for children.
• DICLOFENAC Suppositories 12.5 mg are not used for adults.
3. HOW TO USE DICLOFENAC SUPPOSITORIES
The doctor will tell you how to use Diclofenac Suppositories. Always follow his/her instructions carefully. The dose will be on the pharmacist's label. Check the label carefully. If you are not sure, ask your doctor or pharmacist. Keep using the suppositories for as long as you have been told, unless you have any problems. In that case, check with your doctor.
Suppositories are designed for insertion into the back passage (rectum). Never take them by mouth.
The doctor may also prescribe another drug to protect the stomach to be taken at the same time, particularly if you have had stomach problems before, or if you are elderly, or taking certain other drugs as well.
Adults
Diclofenac Suppositories are normally inserted one, two or three times a day up to a maximum total daily dose of 150mg. The number of suppositories you need will depend on the strength which the doctor has given you.
Elderly
Your doctor may advise you to take a dose that is lower than the usual adult dose if you are elderly. Your doctor may also want to check closely that the Diclofenac Suppositories are not affecting your stomach, particularly during the first 4 weeks that you are using the suppositories.
For the treatment of chronic juvenile arthritis in children aged 1 to 12:
Doses vary with age, but are usually between 1 and 3 mg/kg body weight every day divided into 2 or 3 doses.
For the treatment of post-operative pain in children aged 6 and over:
Doses vary with age, but are usually between 1 and 2 mg/kg body weight per day divided into 2 or 3 doses for no more than 4 days. Your child's doctor will work out the dose that is suitable for your child and will tell you how many Diclofenac Suppositories to use and how often. Follow his/her instructions carefully. If you are not sure about the dose, check with your doctor or pharmacist.
How to insert the suppositories
• Empty your bowels before inserting a suppository.
• Wash your hands.
• Take out the strip of suppositories and tear off one along the perforation.
• Then take the suppository out of the plastic wrapping by pulling back the loose end.
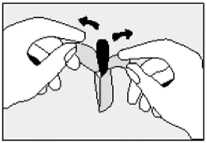
• Lie on one side with your knees pulled up towards your chest.
• Gently push the suppository pointed end first into your back passage (rectum) with your finger. Push the suppository in as far as possible as shown in the diagram.
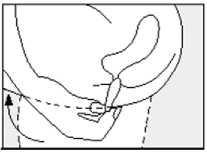
• Lower your legs and, if possible, stay still for a few minutes.
• If you feel as if you need to push the suppository out, try to resist this by lying still with your buttocks pressed together. It is important to keep the suppository in the rectum to allow it to melt and the medicine to be absorbed. Pushing the suppository high into the rectum with your finger will help to reduce this feeling.
• Wash your hands.
The procedure is the same for a child. Once they have emptied their bowels, get them to lie down on their front or side. Gently push the suppository into the child's back passage until it disappears. Try and stop the child moving around for a few minutes to reduce the risk of the suppository coming out.
What if you forget to take a dose?
If you forget to use a suppository, do not worry. Use one as soon as you remember. If it is nearly time for your next dose though, just take the next dose and forget about the one you missed. Do not double up the next dose to make up for the one you missed. Do not insert 2 suppositories at the same time. The total dose should not be more than 150 mg each day if you are an adult. Children should not take more than the dose that is prescribed by their doctor.
What if you use too many suppositories?
You should not take more than 150 mg in one day if you are an adult. Children should not take more than the dose that is prescribed by their doctor. If you accidentally use too many suppositories or use them too often, tell your doctor or go to your nearest casualty department straight away.
4. POSSIBLE SIDE EFFECTS
Diclofenac Suppositories are suitable for most people, but, like all medicines, they can sometimes cause side effects. Side effects may be minimised by using the lowest effective dose for the shortest duration necessary.
Some side effects can be serious
Stop using the suppositories and tell your doctor straight away if you notice:
• Stomach pain, indigestion, heartburn, wind, nausea (feeling sick) or vomiting (being sick)
• Any sign of bleeding in the stomach or intestine, for example, when emptying your bowels, blood in vomit or black, tarry faeces
• Allergic reactions which can include skin rash, itching, bruising, painful red areas, peeling or blistering
• Wheezing or shortness of breath (bronchospasm)
• Swollen face, lips, hands or fingers
• Yellowing of your skin or the whites of your eyes
• Persistent sore throat or high temperature
• An unexpected change in the amount of urine produced and/or its appearance.
If you notice that you are bruising more easily than usual or have frequent sore throats or infections, tell your doctor.
Diclofenac Suppositories may also occasionally cause itching or burning in your back passage or make any haemorrhoids (piles) worse.
The side effects listed below have also been reported.
Common side effects (These may affect between 1 and 10 in every 100 patients):
• Stomach pain, heartburn, nausea, vomiting, diarrhoea, indigestion, wind, loss of appetite
• Headache, dizziness, vertigo
• Skin rash or spots
• Raised levels of liver enzymes in the blood
• Irritation where the suppository is inserted.
• Stomach ulcers or bleeding (there have been very rare reported cases resulting in death, particularly in the elderly)
• Gastritis (inflammation, irritation or swelling of the stomach lining)
• Vomiting blood
• Diarrhoea with blood in it or bleeding from the back passage
• Black, tarry faeces or stools
• Drowsiness, tiredness
• Hypotension (low blood pressure, symptoms of which may include faintness, giddiness or light headedness)
• Skin rash and itching
• Fluid retention, symptoms of which include swollen ankles
• Liver function disorders, including hepatitis and jaundice Very rare side effects (These may affect less than 1 in every 10,000 patients):
Effects on the nervous system:
Tingling or numbness in the fingers, tremor, visual disturbances such as blurred or double vision, hearing loss or impairment, tinnitus (ringing in the ears), sleeplessness, nightmares, mood changes, depression, anxiety, mental disorders, disorientation and loss of memory, fits, headaches together with a dislike of bright lights, fever and a stiff neck, disturbances in sensation.
Effects on the stomach and digestive system:
Constipation, inflammation of the tongue, mouth ulcers, inflammation of the inside of the mouth or lips, taste changes, lower gut disorders (including inflammation of the colon, or worsening of ulcerative colitis or Crohn's disease).
Effects on the heart, chest or blood:
Palpitations (fast or irregular heart beat), chest pain, hypertension (high blood pressure), inflammation of blood vessels (vasculitis), inflammation of the lung (pneumonitis), heart disorders, including congestive heart failure or heart attack, blood disorders (including anaemia).
Effects on the liver or kidneys:
Kidney or severe liver disorders including liver failure, presence of blood or protein in the urine.
Effects on skin or hair:
Serious skin rashes including Stevens-Johnson syndrome, Lyell's syndrome and other skin rashes which may be made worse by exposure to sunlight.
Hair loss.
Other side effects that have also been reported include:
Inflammation of the pancreas, impotence. Facial swelling, inflammation of the lining of the brain (meningitis), stroke, throat disorders, confusion, hallucinations, malaise (general feeling of discomfort), inflammation of the nerves in the eye.
Do not be alarmed by this list - most people use Diclofenac Suppositories without any problems.
If any of the symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor. He/she may want to give you a different medicine.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE DICLOFENAC SUPPOSITORIES
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Diclofenac Suppositories should not be stored above 30°C. Protect from heat.
• Do not use after the expiry date printed on the carton label or blister strip.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Diclofenac Suppositories contain
• Each suppository contains 50mg of the active ingredient diclofenac sodium.
• Diclofenac suppositories also contain the following inactive ingredients: hard fat.
What Diclofenac Suppositories look like and contents of the pack
Diclofenac suppositories are white to yellowish, torpedo-shaped, with smooth surfaces and a slightly fatty odour.
Diclofenac suppositories are available as packs of 10.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1Dx.
Manufacturer
This product is manufactured by
• Famar ABE, Peristeriou, Greece.
• Famar ABE Factory 49 km Athens-Lamia 190 11 Avlona, Greece.
| POM | PL No: 19488/0408 Leaflet revision date: 21 March 2014
Diclofenac is a registered trade mark of Novartis AG, Switzerland.
S408 LEAFLET Dclofenac 20140321