Voltarol Ampoules 75Mg/3Ml
lb NOVARTIS
For the Medical and Pharmaceutical Professions
Dosage and Administration
Adults.-
Voltarol ampoules (given im or iv) should not be given for more than two days; if necessary, treatment can be continued with Voltarol Tablets or Suppositories.
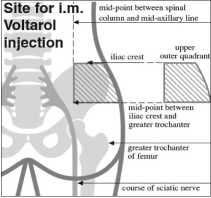
Intramuscular injection: The following directions for intramuscular injection must be adhered to in order to avoid damage to a nerve or other tissue at the injection site.
One ampoule once (or in severe cases twice) daily intramuscularly by deep intragluteal injection into the upper outer quadrant. If two injections daily are required it is advised that the alternative buttock be used for the second injection. Alternatively, one ampoule of 75 mg can be combined with other dosage forms of Voltarol (tablets or suppositories) up to the maximum daily dosage of 150 mg.
Renal colic: One 75 mg ampoule intramuscularly. A further ampoule may be administered after 30 minutes if necessary. The recommended maximum daily dose of Voltarol is 150 mg.
Recommended injection procedure
1. The patient may lie down or stand (holding a stable piece of furniture for support) whichever is most comfortable.
2. The buttocks should be exposed and inspected to find the most suitable injection site. Avoid scars and lumps and choose the buttock which is free from any problems. If more than one injection needs to be given the other buttock should be used.
3. The injection site should be thoroughly disinfected e.g. with isopropyl alcohol and allowed to dry before injecting the solution.
4. Give the deep intramuscular injection high into upper outer quadrant (for boundary definitions see diagram) of the buttock taking particular care to avoid the sciatic nerve (see diagram) and blood vessels (see point 5 below). Avoid injecting into an area where resistance is felt.
N.B. In obese patients avoid deposition of the drug into the
subcutaneous fatty tissue.
In small thin patients with little muscle bulk, be especially aware of
the sciatic nerve which may be quite superficial.
5. Before injection and after needle insertion, pull back the syringe plunger to check the needle has not entered a vessel. If blood is drawn, withdraw the needle to another site and check again.
6. The injection should be given slowly to minimise local tissue damage.
7. If the patient complains of severe pain or pronounced discomfort stop the injection immediately. Retry at another site. A dull aching pain may be experienced after normal injection.
8. Advise the patient to remain reasonably mobile for one to two hours after the injection, whenever possible.
lb NOVARTIS
VOLTAROL® Ampoules
(diclofenac sodium)
Patient Information Leaflet
What you need to know about Voltarol Ampoules
Your doctor has decided that you need this medicine to help treat your condition.
Please read this leaflet carefully before you start to take your medicine. It contains important information. Keep the leaflet in a safe place because you may want to read it again.
If you have any other questions, or if there is something you don’t understand, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Voltarol Ampoules are, and what they are used for
2. Things to consider before you start to take Voltarol Ampoules
3. How to take Voltarol Ampoules
6288203
4. Possible side effects
5. How to store Voltarol Ampoules
6. Further information
1. What Voltarol Ampoules are, and what they are used for
Diclofenac sodium, the active ingredient in Voltarol Ampoules, is one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs reduce pain and inflammation.
The intramuscular injection is used to treat a number of painful conditions including:
• 'Flare-ups' of joint or back pain
• Attacks of gout
• Pain caused by kidney stones
• Pain caused by injuries.
Voltarol Ampoules can either be given as an injection into the muscle, or as a slow infusion into a vein. The intravenous infusion is used in hospitals to prevent or treat pain following an operation.
Voltarol Ampoules are not suitable for children.
2. Things to consider before you have Voltarol injection
Some people MUST NOT have this injection. Talk to your doctor if:
• you think you may be allergic to diclofenac sodium, sodium metabisulphite, aspirin, ibuprofen or any other NSAID, or to any of the other ingredients of Voltarol Ampoules. (These are listed at the end of the leaflet.) Signs of a hypersensitivity reaction include swelling of the face and mouth (angioedema), breathing problems, runny nose, skin rash or any other allergic type reaction
2078879
• you have now, or have ever had, a stomach (gastric) or duodenal (peptic) ulcer, or bleeding in the digestive tract (this can include blood in vomit, bleeding when emptying bowels, fresh blood in faeces or black, tarry faeces)
• you have had stomach or bowel problems after you have taken other NSAIDs
• you have moderate or severe heart, kidney or liver failure
• if you have established heart disease and/or cerebrovascular disease e.g. if you have had a heart attack, stroke, mini-stroke (TIA) or blockages to blood vessels to the heart or brain or an operation to clear bypass blockages
• if you have or have had problems with your blood circulation (peripheral arterial disease)
• you are more than six months pregnant
You should also ask yourself these questions before having a
Voltarol Injection or Infusion:
• Do you suffer from any bowel disorders including ulcerative colitis or Crohn's disease?
• Do you have kidney or liver problems, or are you elderly?
• Do you suffer from any blood or bleeding disorder?
• Do you have a condition called porphyria?
• Have you ever had asthma?
• Are you breastfeeding?
• Do you have angina, blood clots, high blood pressure, raised cholesterol or raised triglycerides
• Do you have heart problems, or have you had a stroke, or do you think you might be at risk of these conditions (for example, if you have high blood pressure, diabetes or high cholesterol or are a smoker)?
• Do you have diabetes
• Do you smoke
• Do you have Lupus (SLE) or any similar condition?
• Could you be suffering from dehydration?
• Have you suffered any heavy loss of blood recently?
If the answer to any of these questions is YES, discuss your treatment with your doctor or pharmacist because Voltarol Ampoules might not be the right medicine for you.
Are you taking other medicines?
Some medicines can interfere with your treatment. Tell your doctor or pharmacist if you are taking any of the following:
• Medicines to treat diabetes
• Anticoagulants (blood thinning tablets like warfarin)
• Diuretics (water tablets)
• Lithium (used to treat some mental problems)
• Methotrexate (for some inflammatory diseases and some cancers)
• Ciclosporin and tacrolimus (used to treat some inflammatory diseases and after transplants)
• Trimethoprim (a medicine used to prevent or treat urinary tract infections)
• Quinolone antibiotics (for infections)
• Any other NSAID or COX-2 (cyclo-oxgenase-2) inhibitor, for example aspirin or ibuprofen
• Mifepristone (a medicine used to terminate pregnancy)
• Cardiac glycosides (for example digoxin), used to treat heart problems
• Medicines known as SSRIs used to treat depression
• Oral steroids (an anti-inflammatory drug)
• Medicines used to treat heart conditions or high blood pressure, for example beta-blockers or ACE inhibitors.
• Voriconazole (a medicine used to treat fungal infections).
• Phenytoin (a medicine used to treat seizures)
• Colestipol/cholestyramine (used to lower cholesterol)
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Pregnancy
• Are you pregnant or planning to become pregnant? Although not common, abnormalities have been reported in babies whose mothers have taken NSAIDs during pregnancy. You should not have a Voltarol Injection during the last 3 months of pregnancy as it may affect the baby’s circulation.
• Are you trying for a baby? Having Voltarol Injections may make it more difficult to conceive. You should talk to your doctor if you are planning to become pregnant, or if you have problems getting pregnant.
Will there be any problems with driving or using machinery?
Very occasionally people have reported that Voltarol Ampoules have made them feel dizzy, tired or sleepy. Problems with eyesight have also been reported. If you are affected in this way, you should not drive or operate machinery.
Other special warnings
• You should take the lowest dose of Voltarol for the shortest possible time, particularly if you are underweight or elderly.
• There is a small increased risk of heart attack or stroke when you are taking any medicine like Voltarol. The risk is higher if you are taking high doses for a long time. Always follow the doctor’s instructions on how much to take and how long to take it for.
• Whilst you are taking these medicines your doctor may want to give you a check-up from time to time.
• If you have a history of stomach problems when you are taking NSAIDs, particularly if you are elderly, you must tell your doctor straight away if you notice any unusual symptoms. 925770 gb lft
• Because it is an anti-inflammatory medicine, Voltarol may reduce the symptoms of infection, for example, headache and high temperature. If you feel unwell and need to see a doctor, remember to tell him or her that you are taking Voltarol.
• Voltarol Ampoules contain the preservative, sodium metabisulphite. This can sometimes cause allergic reactions and breathing difficulties.
• Voltarol Ampoules should not be used in children.
3. How to take Voltarol Ampoules
Your doctor will decide when and how to treat you with Voltarol Ampoules. You will either be given an intravenous infusion (a drip into a vein) or an intramuscular injection (an injection into a muscle). The intramuscular injection is usually injected into the buttocks.
The usual dose is:
Adults
One or two ampoules (75 to 150 mg) each day for one or two days. Elderly
Your doctor may give you a dose that is lower than the usual adult dose if you are elderly.
Children
Not suitable for children.
A doctor, nurse or pharmacist will prepare the injection for you.
If you have had an operation and are in hospital, the ampoule contents may be diluted and put into a drip bag before being given to you. A nurse or doctor will usually then give you the injection or infusion. You would not usually have to give the injection to yourself.
The doctor may also prescribe another drug to protect the stomach to be taken at the same time, particularly if you have had stomach problems before, or if you are elderly, or taking certain other drugs as well.
What if you have had too much Voltarol? (Overdose)
If you think you have been given too much Voltarol tell your doctor or nurse straight away.
4. Possible side effects
Voltarol Ampoules are suitable for most people, but, like all medicines, they can sometimes cause side effects. Side effects may be minimised by using the lowest effective dose for the shortest duration necessary.
Some side effects can be serious
Tell the doctor straight away if you notice:
• Stomach pain, indigestion, heartburn, wind, nausea (feeling sick) or vomiting (being sick)
• Any sign of bleeding in the stomach or intestine, for example, when emptying your bowels, blood in vomit or black, tarry faeces
• Allergic reactions which can include skin rash, itching, bruising, painful red areas, peeling or blistering
• Wheezing or shortness of breath (bronchospasm)
• Swollen, face, lips, hands or fingers
• Yellowing of your skin or the whites of your eyes
• Persistent sore throat or high temperature
• An unexpected change in the amount of urine produced and/or its appearance.
If you notice that you are bruising more easily than usual or have frequent sore throats or infections, tell your doctor.
The side effects listed below have also been reported.
Common side effects (These may affect between 1 and 10 in every 100 patients):
Stomach pain, heartburn, nausea, vomiting, diarrhoea, indigestion, wind, loss of appetite
Headache, dizziness, vertigo Skin rash or spots
Raised levels of liver enzymes in the blood
Injection site reactions, symptoms include redness, swelling, change in
the skin colour, inflammation, pain, and hypersensitivity
Rare side effects (These may affect between 1 in every 1000 to 1 in every 10,000 patients):
Stomach ulcers or bleeding (there have been very rare reported cases resulting in death, particularly in the elderly)
Gastritis (inflammation, irritation or welling of the stomech lining) Vomiting blood
Diarrhoea with blood in it or bleeding from the back passage Black, tarry faeces or stools Drowsiness, tiredness
Hypotension (low blood pressure, symptoms of which may include faintness, giddiness or light headedness)
Skin rash and itching
Fluid retention, symptoms of which include swollen ankles
Liver function disorders, including hepatitis and jaundice
Injection site necrosis (dead skin and tissue around the injection site)
Very rare side effects (These may affect less than 1 in every 10,000 patients):
Effects on the nervous system:
Tingling or numbness in the fingers, tremor, visual disturbances such as blurred or double vision, hearing loss or impairment, tinnitus (ringing in the ears), sleeplessness, nightmares, mood changes, depression, anxiety, mental disorders, disorientation and loss of memory, fits, headaches together with a dislike of bright lights, fever and a stiff neck, disturbances in sensation.
Effects on the stomach and digestive system:
Constipation, inflammation of the tongue, mouth ulcers, inflammation of the inside of the mouth or lips, taste changes, lower gut disorders (including inflammation of the colon, or worsening of colitis or Crohn's disease).
Effects on the heart, chest or blood:
Palpitations (fast or irregular heart beat), chest pain, hypertension (high blood pressure), inflammation of blood vessels (vasculitis), inflammation of the lung (pneumonitis), heart disorders, including congestive heart failure or heart attack, blood disorders (including anaemia).
Effects on the liver or kidneys:
Kidney or severe liver disorders including liver failure, presence of blood or protein in the urine.
Effects on skin or hair:
Serious skin rashes including Stevens-Johnson syndrome Lyell's syndrome and other skin rashes which may be made worse by exposure to sunlight.
Hair loss.
Other side effects that have also been reported include:
Inflammation of the pancreas, impotence. Facial swelling, inflammation of the lining of the brain (menigitis), stroke, throat disorders, confusion, hallucinations, malaise (general feeling of discomfort), inflammation of the nerves in the eye.
Do not be alarmed by this list - most people have an injection of Voltarol without any problems.
If any of the symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor. He/she may want to give you a different medicine.
5. How to store Voltarol Ampoules
Store below 30°C. Protect from light and heat.
Keep out of the reach and sight of children.
Do not use Voltarol Ampoules after the expiry date which is printed on the outside of the pack.
6. Further information
The glass ampoules contain 75 mg of the active ingredient, diclofenac sodium, in solution.
The ampoules also contain mannitol, sodium metabisulphite (E223), benzyl alcohol, propylene glycol, sodium hydroxide, water.
Voltarol Ampoules come in packs of 10.
The Product licence holder is Novartis Pharmaceuticals UK Limited, Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR, England.
Voltarol Ampoules are manufactured by Novartis Pharmaceuticals UK Ltd, Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, United Kingdom.
This leaflet was revised in September 2013
If you would like any more information, or would like the leaflet in a different format, please contact Medical Information at Novartis Pharmaceuticals UK Ltd, telephone number 01276 698370.
VOLTAROL is a registered trade mark Copyright Novartis Pharmaceuticals UK Limited
Intravenous Infusion: Immediately before initiating an intravenous infusion, Voltarol must be diluted with 100-500 ml of either sodium chloride solution (0.9%) or glucose solution (5%). Both solutions should be buffered with sodium bicarbonate solution (0.5 ml 8.4% or 1 ml 4.2%). Only clear solutions should be used.
Intravenous infusions should be freshly made up and used immediately. Once prepared, the infusion should not be stored.
Voltarol must not be given as an intravenous bolus injection.
Two alternative regimens are recommended: For the treatment of moderate to severe post-operative pain, 75 mg should be infused continuously over a period of 30 minutes to 2 hours. If necessary, treatment may be repeated after 4-6 hours, not exceeding 150 mg within any period of 24 hours.
For the prevention of post-operative pain, a loading dose of 25 mg-50 mg should be infused after surgery over 15 minutes to 1 hour, followed by a continuous infusion of approx. 5mg per hour up to a maximum daily dosage of 150 mg.
Children:
Voltarol ampoules are not recommended for use in children.
Elderly: Although the pharmacokinetics of Voltarol are not impaired to any clinically relevant extent in elderly patients, non-steroidal anti-inflammatory drugs should be used with particular caution in such patients who generally are more prone to adverse reactions. In particular it is recommended that the lowest effective dosage be used in frail elderly patients or those with a low body weight (see also Precautions) and the patient should be monitored for GI bleeding for 4 weeks following initiation of NSAID therapy.
The recommended maximum daily dose of Voltarol is 150 mg.
Incompatibilities
The ampoules used im or iv as an infusion should not be mixed with other injection solutions.
Shelf life
Two years.
Special precautions for storage
Protect from light and heat (store below 30°C).
Medicines should be kept out of the reach of children.
The infusion solution should not be used if crystals or precipitates are observed.
Nature and contents of container
The glass ampoules (Ph.Eur. Type I) contain colourless to faintly yellow liquid and come in packs of 10.
925770 GB LFT