Voltarol Ophtha 0.1% Eye Drops
Out of date information, search anotherPATIENT INFORMATION LEAFLET 2070
02.10.10[2]
Voltarol® Ophtha 0.1% Eye Drops
(diclofenac sodium)
Your doctor has decided that you need these eye drops to help treat your condition.
Please read this leaflet carefully before you start to use the eye drops. It contains important information. Keep the leaflet in a safe place because you may want to read it again.
If you have any other questions, or if there is something you don't understand, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
The eye drops will be referred to as Voltarol Ophtha in this leaflet.
In this leaflet:
1. What Voltarol Ophtha are and what they are used for
2. Things to consider before you start to use Voltarol Ophtha
3. How to use Voltarol Ophtha
4. Possible side effects
5. How to store Voltarol Ophtha
6. Further information
1. What Voltarol Ophtha are and what they are used for
Voltarol Ophtha contain 0.1% of the active ingredient diclofenac sodium. Diclofenac sodium is one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs reduce pain and inflammation.
Voltarol Ophtha are used
• before eye surgery, to help keep the pupil open during surgery, or
• after eye surgery or injury, to control pain and/or inflammation,
• to reduce the symptoms, such as red, runny or itchy eyes associated with seasonal allergic conjunctivitis (hayfever).
2. Things to consider before you have Voltarol Ophtha
Some people MUST NOT have Voltarol Ophtha. Talk to your doctor if:
• you think you may be allergic to diclofenac sodium, aspirin or any other NSAIDs, or to any of the other ingredients of the eye drops. (These are listed at the end of the leaflet.)
• You are more than 6 months pregnant.
You should also ask yourself these questions before you have Voltarol Ophtha:
• Do you have an eye infection?
• Are you using any other eye drops?
• Do you have any bleeding disorders?
• Are you pregnant or breast feeding?
If the answer to any of these questions is YES, tell your doctor or pharmacist because Voltarol Ophtha might not be the right medicine for you.
Are you taking other medicines?
Voltarol Ophtha may interfere with other drugs you might be taking or using.
Tell your Doctor if you are taking or using:
• Medicines to prevent your blood clotting.
• Eye drops or ointment containing steroids such as hydrocortisone or betamethasone.
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Will there be any problems with driving or using machinery?
Some people may have problems with their eyes such as blurred vision, while they are being treated with Voltarol Ophtha. If you are affected, you should not drive or use machinery.
Other special warnings
• The eye drops contain polyoxyl 35 castor oil which can sometimes cause skin reactions.
• If you are using more than one sort of eye drops leave five minutes between applications.
3. How Voltarol Ophtha is used
When Voltarol Ophtha is being used during eye surgery the doctor will work out the correct dose.
If you have been prescribed the drops to use at home the doctor will tell you how and when to use them. Always follow the doctor's instructions carefully.
The usual dose is 1 drop in the affected eye 4 times a day.
How often you use the drops, and how long you use them for, will be different depending on your condition.
The dose will be on the pharmacist's label. Check the label carefully. If you are not sure, ask your doctor or pharmacist.
Instructions for using the eye drops are given at the end of this leaflet.
These drops should not be used in children under 18 years old.
What if you forget to use the drops?
If you miss a dose continue with the next dose as normal. Do not double the dose to make up for the one you missed.
What if you use too much?
If you use too much or if you accidentally swallow the eye drops, see your doctor at once or go to your nearest hospital casualty department. Take your medicine with you.
4. Possible side effects
Voltarol Ophtha are suitable for most people, but, like all medicines, they can sometimes cause side effects.
Stop using Voltarol Ophtha and tell your doctor straight away if you notice:
• Allergic reactions in your eyes such as red, itching and swollen eyes and eyelids.
• Other general allergic reaction symptoms such as rash, redness, itching, cough and runny or stuffy nose.
The following side effects have also been reported:
• Eye pain.
• A mild or moderate burning sensation and/or redness in the eyes.
• Blurred vision immediately after using the eye drops.
• Breathlessness and wheezing or other symptoms of asthma.
• Unusual sensitivity to light.
• Damage to cells on the surface of the cornea (the membrane covering the front of the eye), corneal thinning or ulcers that might result in loss of sight.
If any of these symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor. He/she may want to give you a different medicine.
5. How to store Voltarol Ophtha
Keep all medicines out of the reach and sight of children.
Do not store above 25°C.
The drops are sterile until the tab is removed.
Do not use more than 28 days after opening the blister pack.
Do not use the drops after the expiry date which is printed on the outside of the pack.
If your doctor tells you to stop using Voltarol Ophtha, please take any left back to your pharmacist to be destroyed. Only keep the drops if the doctor tells you to. Do not throw them away with your normal household water or waste. This will help to protect the environment.
6. Further information
Voltarol Ophtha contain 0.1% (1 mg/ml) of the active ingredient, diclofenac sodium.
They also contain the inactive ingredients boric acid, polyoxyl 35 castor oil, trometamol and water for injections.
Voltarol Ophtha are single dose unit vials containing 0.3 ml of a clear, colourless and odourless solution sufficient for one eye. They come in packs of 5, 10 and 40.
Marketing Authorisation Holder and Manufacturer
Manufactured by Novartis Norge AS, Brynsalleen 4, Oslo, N-0667 Norway. Procured from within the EU by the Parallel Import Product Licence holder Tenolol Ltd, 5 Sandridge Close, Harrow, Middlesex HA1 1XD. Repackaged by Servipharm Ltd.
| POM | | PL 30900/2070
How to use Voltarol Ophtha single dose units
• Wash your hands before applying your drops.
• Open blister pack and remove the strip of single dose units.
• Tear off one single dose unit from the strip (SEE FIGURE 1). Hold it by the small tab with the nozzle pointing downwards and tap the single dose unit gently until all the air bubbles are above the solution.
• Twist the tab off the single dose unit (SEE FIGURE 2).
• Make sure that the tip of the single dose unit does not touch anything.
• Hold the single dose unit in one hand between the thumb and forefinger.
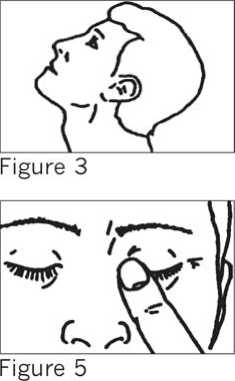
• With your head tilted back (SEE FIGURE 3) use the forefinger of your other hand to pull down the lower lid of your eye.
• Place the single dose unit tip close to your eye, but without touching the eye or lid, and gently squeeze the single dose unit to allow one drop to be applied (SEE FIGURE 4).
• Close your eyelid and gently press the inner corner of your eye with your forefinger for three minutes (SEE FIGURE 5).
• Discard the single dose unit and remaining solution and wash your hands.
Follow these instructions carefully. Consult your doctor or pharmacist if
there is anything you do not understand.
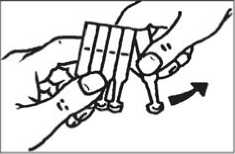
Figure 1
|
A | |
|
Figure 2 | |
Date of revision of leaflet: 02.10.10[2]
Voltarol is a registered trademark of Novartis AG.