Water For Injections
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Water for Injections
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Water for Injection BP has the following composition:
|
Name |
Specification Reference |
%w/v |
|
Water for Inections in bulk |
EP |
100 |
3. PHARMACEUTICAL FORM
A sterile parenteral diluent.
4. CLINICAL PARTICULARS
4.1 Therapeutic Indications
For use as a diluent only. Thereafter by any parenteral route.
4.2 Posology and method of administration
The volume to be used is determined by the physician, pharmacist or nurse.
4.3 Contraindications
None.
4.4 Special warnings and special precautions for use
Not to be used alone as an intravenous injection.
Contains no added bactericide.
If the whole of the content of this container is not used immediately after the seal has been broken, the remainder should be discarded.
The label states: For use as a diluent.
4.5 Interaction with other medicinal products and other forms of interaction
None.
4.6 Fertility, pregnancy and lactation
Although not intended to be used alone as an intravenous injection, the use of water for injections during pregnancy and lactation is not considered likely to constitute a hazard.
4.7 Effects on ability to drive and use machines
Not applicable.
4.8 Undesirable effects
None
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Not applicable.
PHARMACOLOGICAL PROPERTIES
5.
5.1 Pharmacodynamic properties
N/A
5.2 Pharmacokinetic properties
N/A
5.3 Preclinical safety data
N/A
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
None.
6.2 Incompatibilities
None.
6.3 Shelf life
500 & 1000ml Polyethylene container - 60 months
500 & 1000ml Polyolefin bag - 36 months
5ml, 10ml and 20ml Polyethylene ampoules - 24 months
500 & 1000ml Polyethylene bottle with cap and administration/addition points - 36 months
6.4 Special precautions for storage
None.
6.5 Nature and contents of container
Colourless ampoule of non-toxic polythene (500ml or 1000ml)
or
A flexible 500 or 1000ml polyolefine bag sealed in a polyolefine overwrap.
Or
Polyethylene ampoule LDPE (Low density polyethylene), 3220D (5ml or 10ml and 20ml)
Box with 20 ampoules of 5 ml Box with 20 ampoules of 10 ml Box with 20 ampoules of 20 ml Or
Polyethylene bottle (500 or 1000 ml) with a cap with an administration point and an addition point (KabiPac).
6.6 Special precautions for disposal
Opening the overwrap:
Locate the corner tabs at the end of the bag. Grip the two tabs and pull the two halves of the overwrap apart, releasing the bag onto a clean surface.
Setting up the solution:
Position the roller clamp of the giving-set to just below the drip chamber and close.
Hold the base of the giving set port firmly and grip the wings of the twist of tab.
Twist to remove the protective cover.
Still holding the base of the giving-set port push the set spike fully into the port to ensure a leak proof connection.
Prime the set in accordance with the manufacturer’s instructions.
5ml, 10ml and 20ml ampoules
To open:
Shake the ampoule as shown below in order to remove the liquid kept in the cap (1a, 1b). Pull the tab as indicated by the arrow, forward (2) and backward (3), and then twist (4).
Discard the tab (5).
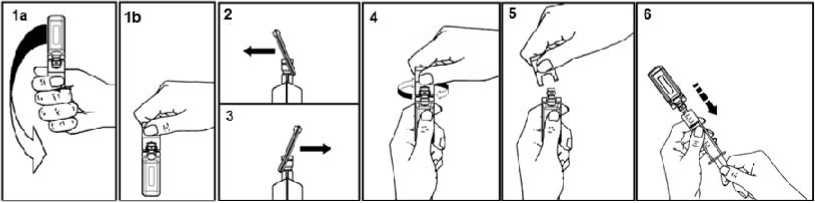
After opening the ampoule, its spout is perfectly adapted to the Luer syringe and Luer-Lock. Therefore, no needle is needed to extract the solution.
Connect the syringe to the injectable using a rotating movement. Extract the liquid (6).
7 MARKETING AUTHORISATION HOLDER
Fresenius Kabi Limited
Cestrian Court
Eastgate Way
Manor Park
Runcorn
Cheshire
WA7 1NT
8. MARKETING AUTHORISATION NUMBER
PL 8828\0047
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
03.89/10.99
10 DATE OF REVISION OF THE TEXT
07/10/2014