Water For Injections
B306139/00
PACKAGE LEAFLET: INFORMATION FOR THE USER
Water for Injections
Read all of this leaflet carefully before you
start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell you doctor or pharmacist.
In this leaflet:
1. What Water for Injections is and what it is used for
2. Before you are given Water for Injections
3. How you are given Water for Injections
4. Possible side effects
5. How Water for Injections is stored
6. Further information
1. WHAT WATER FOR INJECTIONS IS AND WHAT IT IS USED FOR
Water for Injections is contained in a sealed clear ampoule or bag.
Water for Injections is a diluent. This means that it is used where sterile water is needed to dilute drugs that will then be injected.
2. BEFORE YOU TAKE WATER FOR INJECTIONS
Take special care with Water for Injections
Your doctor will know how to use Water for injections.
The water is not injected alone and is used only for dilutions. Water for Injections contains no chemicals to prevent bacteria and should be used immediately after the seal is broken.
Any unused water should be thrown away. Your doctor or nurse will ensure the water is clear and free from particles before use.
Taking other medicines
Water for Injections does not interfere with other medicines.
Pregnancy and breast-feeding
Water for Injections does not have any effect during pregnancy or breast-feeding.
Driving and using machines
Water for Injections has no effect on driving or using machines.
3. HOW TO TAKE WATER FOR INJECTIONS
Water for Injections will be given to you in hospital. The amount used will be calculated by the doctor, pharmacist, or nurse.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
There are no known side effects, but if you think you are having a side effect, please tell your doctor or pharmacist.
5. HOW TO STORE WATER FOR INJECTIONS
Keep out of the reach and sight of children. Your doctor and hospital pharmacist are responsible for the correct storage, use and disposal of Water for Injections.
The infusion requires no special storage conditions.
The solution must not be used after the expiry date shown on the label. The expiry date refers to the last day of that month.
Handling instructions:
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liquid kept in the cap (2). To open the ampoule, twist the ampoule body and ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
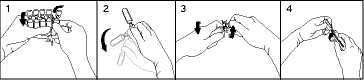
Therefore, no needle is needed to extract the solution. Extract the liquid.
6. FURTHER INFORMATION
What Water for Injections contain:
Water for Injections BP.
What Water for Injections looks like and contents of the pack.
Water for Injections are colourless bottles of polyethylene known as either Polyfusor® or Kabipac® (500 ml or 1000 ml) or
A flexible 500 or 1000 ml bag sealed in an
overwrap
or
Polyethylene ampoules (5 ml or 10 ml and 20 ml)
Box with 20 ampoules of 5 ml
Box with 20 ampoules of 10 ml
Box with 20 ampoules of 20 ml
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Fresenius Kabi Limited Cestrian Court, Eastgate Way,
Manor Park, Runcorn,
Cheshire, WA7 1NT. UK.
Manufacturer:
FRESENIUS KABI Spain, S.A.
C/ Marina, 16-18, planta 17 08005-Barcelona, Spain.
This leaflet was last approved in September 2009
V001/ES