Wilko Max Strength Echinacea Cold And Flu Relief
Out of date information, search anotherBrunei
Healthcare
MANUFACTURING
Packing Standard for Pharmacode on Leaflet
Line 7
NPI VER 001 JAN 2010
FONT SIZE 8pt
Echinacea
T4mm
F1T339"'
5mm
Echinacea purpurea root extract
TEXT
FREE
AREA
18mm
19/3/12
Pantone 3145C
Read all of this leaflet carefully before you take this product because it contains important information -you 'need' to' know:......................
This product is available without prescription; however, you still need to use this product carefully to get the best results from it. Keep this leaflet as you may need to read it again. Ask your doctor, pharmacist or healthcare practitioner if you need more information or advice. You must contact a doctor if your symptoms worsen or do not improve after 10 days. If any of the side effects get serious or if you notice any side effects not listed in this leaflet, please tell your doctor.
In this leaflet:
I. What the product is and what it is used for
2: Before you take this product
3: How to take this product
4: Possible side effects
5: How to store this product
6: Further information
or if you suffer from:
• frequent allergic reactions such as hives
........(urticaria); eczema; asthma................
• the infection: tuberculosis
• connective tissue disease with formation of clumps of cells (sarcoidosis) mainly occurring in the lymph nodes, lungs and liver
• auto-immune diseases such as inflammation of the connective tissue (collagenosis) or multiple sclerosis
• conditions which decrease your natural response to infection (eg HIV infection or AIDS)
• are on therapy to reduce your natural response to infection (immunosuppression eg: chemotherapy or radiotherapy for cancer;

1. What is this product and what is it used for?
This product contains:
• Echinacea purpurea root extract
These tablets are a traditional herbal medicinal product used to relieve the symptoms of the common cold and influenza type infections based on traditional use only.
history" of organ or bone marrow' transplant)
• blood disorders involving the white blood cell system such as low white cell count due to bone marrow disorders (agranulocytosis) or blood cell cancer (leukemias)
or if you are already taking immunosuppressant
medication such as ciclosporin or methotrexate.
Take special care with this product:
• If there is a family history of allergic reactions.
• Because Echinacea can trigger auto-immune diseases.
Taking other medicines:
Please tell your doctor or pharmacist if you are
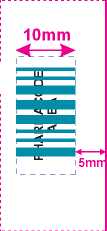
Position pharmacode centrally on fold panel with a 3-5mm distance from the edge of the leaflet.
fold
1st bar
flj
last bar
fold central
Leaflet Size: 150 x 140mm height x width
2. Before you take this product
This product is not suitable for children under 12 years of age.
Do not take this product if you are allergic to:
• Echinacea or products from the same plant family: (Asteraceae/Compositae) such as daisies, marigolds or artichokes
• any of the other ingredients of this product. (See section 6 further information).
' taking"me'dicines " obtained" without" a" prescription. P reg n ancy and breast feeding Do not take this product if you are pregnant or breast feeding, because there is no evidence that it is safe to do so.
3. How to take this product
Start at the first signs of a common cold. Swallow the tablet whole with water.
Do not chew.
5mm
fold
FRONT
Leaflet fold to: 140mm x 39mm with 2mm Lip withTitle showing on side and
Pharmacode showing on the other
TEXT FREE AREA ^ 18mm
Adults, elderly and children over 12 years:
Take 1 tablet, three times a day, if required.
Do not give to children under 12 years:
Do not exceed the stated dose:
Do not take this product for more than 10 days. If the condition worsens or high fever occurs whilst taking the product or if symptoms persist for more than 10 days, see a doctor or pharmacist.
If you take too many tablets by mistake, contact your doctor or pharmacist straight away.
5. How to store this product
4mm
Keep out of the reach and sight of children:
Do not use these tablets after the expiry date printed on the pack.
Do not store above 25oC. Store in the original container.
6. Further information
4. Possible side effects
V
-►
Direction ofTravel
Like all medicines, this product can cause side effects, although not everybody gets them.
The frequency of the side effects is not known.
The possible side effects include:
• allergic reactions such as swelling, hives, or rashes
• swelling of the skin due to fluid
• swelling of the facial area (Quinke's oedema)
• shrinking of the airways of the lungs with obstruction (bronchospasm)
• asthma and life threatening reactions (anaphylactic shock).
■Echinacea" can trigger allergic" reactions "in patients"' who have a tendency to allergic reactions. Stop taking the product immediately if you experience any allergic reaction.
Association with auto-immune diseases has been reported such as:
• inflammation of the brain and spinal chord (disseminated encephalitis)
• painful lumps on the shins (erythema nodosum)
• low blood platelet count (immunothrombocytopenia)
• destruction of blood cells by antibodies (Evans Syndrome)
.•.. dryness .in. the mouth and. eye with .kidney____
tubular dysfunction (Sjogren Syndrome).
A decrease in the number of white blood cells (leucopenia) may occur in long term use (more than 8 weeks).
If you are concerned about any side effect, if a side effect becomes serious, or if you notice a side effect not listed in this leaflet, please tell your doctor, or healthcare practitioner.
Each film coated tablet contains the active ingredient: 143.0mg of extract (dry extract) from .E.ch[na_cea_purpurea. root (6-7j1) .Ce9u.ivaJe.nt_ to.... 858 mg - 1000 mg of Echinacea purpurea (L.) Moench, root)
Also contains: maltodextrin, calcium hydrogen phosphate dihydrate, microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silica, magnesium stearate, hypromellose, purified talc, titanium dioxide (E171), yellow iron oxide (E172).
This product is available in pack sizes of 30, 60, 120 and 180 tablets. Not all pack sizes are marketed.
You can help to make medicines safer by reporting any side-effects to the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. Alternatively you can get a paper Yellow Card form from your GP’s surgery or pharmacy, or call freephone 0808 100 3352 (available 10am-2pm Monday - Friday).
To request a copy of this leaflet in braille, large print or audio please call 0800 198 5000 (UK only). Please be ready to give the following:
Product name: HealthAid® EchiGuard™ Cold and Flu Relief
Reference number: THR 20894/0063
THR holder and manufacturer is: Brunel Healthcare Manufacturing Ltd, William Nadin Way, ■Swadlincote; Derbyshire; DETT 0BB; U:K:..........
Text revised: January 2012 THR 20894/0063

Certification Mark
HEALTHAID LTD
HealthAid House, Marlborough Hill, Harrow, Middlesex, HA1 1UD, England
5mm
fold
fold central
Leaflet Size: 150 x 140mm height x width
fold
Other Information
BACK
|
Signature |
Print Name |
Date | |
|
Approved by | |||
|
Packing | |||
|
Engineering |