Willfact 2000 I.u Powder And Solvent For Solution For Injection
Slovak Republic Spain Sweden
United Kingdom This leaflet was last revised in June 2015
Other medicines and Will fact
Tell your doctor or pharmacist if you are taking/using, have recently taken/used or might take/use any other medicines.
Willfact with food and drink
There are no known interactions of VWF preparations with foods or drinks. Therefore you do not have to avoid any specific foods or drinks.
Pregnancy and breast-feeding
Willfact should be used during pregnancy and breastfeeding only if it is clearly indicated. The safety of Willfact during pregnancy and breastfeeding has not been evaluated in controlled clinical studies. Animal studies are not sufficient to establish its safety with respect to fertility, pregnancy and development of the child during pregnancy and after birth.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Driving and using machines
No effects on the ability to drive or use machines have been observed.
Willfact contains sodium
One 5 ml vial (500 IU) of Willfact contains 0.15 mmol (3.4 mg) sodium.
One 10 ml vial (1000 IU) of Willfact contains 0.3 mmol (6.9 mg) sodium.
One 20 ml vial (2000 IU) of Willfact contains 0.6 mmol (13.8 mg) sodium.
You should take it into consideration if you are on a salt-free or low-salt diet.
3. HOWTO USE WILLFACT
Treatment should only be initiated under the supervision of a physician experienced in the treatment of haemostatic disorders.
Dosage
The dose you take depends on your health condition and body weight.
The first dose of Willfact is 40 to 80 lU/kg for the treatment of haemorrhage or trauma, in conjunction with the reguired amount of factor VIII product, calculated according to your baseline plasma level of FVIIkC, in order to achieve an appropriate plasma level of FVIIkC, immediately before the intervention or as soon as possible after the onset of the bleeding episode or severe trauma.
If required, you will receive further doses of Willfact of 40 to 80 lU/kg per day in one or two injections daily over one to several days.
Willfact can also be administered as long-term prophylaxis; the dose level is determined individually in this case. Willfact doses between 40 and 60 lU/kg administered two to three times per week reduce the number of haemorrhagic episodes.
Please talk to your doctor if you feel that the effect of Willfact is too strong or too weak.
If you use more Willfact than you should:
No symptoms of overdose with Willfact have been reported.
However, the risk of thrombosis cannot be excluded in case of major overdose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side-effects occurred "uncommonly”: (may affect up to 1 in 100 people):
Hypersensitivity - or allergic reactions
In some cases, the following signs may progress to severe allergic reaction (anaphylaxis) including shock.
■ Accelerated heartbeat (tachycardia)
■ Tightness of the chest
■ Headache
■ Restlessness
■ Tingling
■ Wheezing
■ Nausea
■ Vomiting
■ Quincke’s oedema (angiooedema)
■ Nettle rash (generalised urticaria)
■ Hives
■ Drop in blood pressure (hypotension)
■ Burning and stinging at the infusion site
■ Chills
■ Flushing/heat
■ Listlessness (lethargy)
The early signs of allergic reactions could be hives, nettle rash, tightness of the chest, wheezing, hypotension and sudden severe allergic reaction. If one of these effects occurs, immediately stop the treatment and alert a doctor to start appropriate treatment depending on the type and severity of the reaction.
The following side-effects were observed "rarely” (may affect up to 1 in 1000 people):
■ Fever
The following side-effects occurred "very rarely” (may affect up to 1 in 10000 people):
Antibodies (inhibitors) against VWF: very rarely, proteins may be formed in patients with von Willebrand disease, especially type 3 patients, which neutralise the effect of VWF. These proteins are called antibodies or inhibitors. However, this has never been observed during Willfact treatment. Patients treated with VWF should be carefully monitored by their doctors for the development of inhibitors by appropriate clinical observations and laboratory tests. If such inhibitors occur, the condition will manifest itself as an inadequate clinical response. The antibodies form antibody-antigen complexes and occur concomitantly to anaphylactic reactions.
After correction of the factor Willebrand deficiency, you must be monitored for early signs of thrombosis or disseminated intravascular coagulation and receive treatment to prevent thrombosis in situations involving an increased risk of thrombosis (after operations, during confinement to bed, in cases of deficiency in a coagulation inhibitor or fibrinolytic enzyme).
If you receive FVIII-containing VWF preparations, the risk of thrombosis may also be increased due to persistently elevated FVIILC plasma levels.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.qov.uk/vellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOWTO STORE WILLFACT
■ Store in the original package in order to protect from light. Do not store above 25°C. Do not freeze.
■ The product should be used immediately after reconstitution. However, its stability has been demonstrated for 24 hours at 25°C.
■ Keep product out of the the sight and reach of children.
■ Do not use the product after the expiry date stated on the vial label and carton.
■ Do not use this medicine if you notice that the solution is cloudy or that it contains any deposit.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist or your nurse how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Willfact contains
The active substance is: human von Willebrand factor (500 IU, 1000 IU or 2000 IU), expressed in International Units (IU) of Ristocetin Cofactor activity (VWF:RCo). After reconstitution with 5 ml (500 IU), 10 ml (1000 IU) or 20 ml (2000 IU) of water for injections, one vial contains approximately 100 lU/ml of human von Willebrand factor.
Before the addition of albumin, the specific activity is greater than or equal to 50 IU of VWF: RCo/mg of total protein.
The other ingredients are:
Powder: human albumin, arginine hydrochloride, glycine, sodium citrate and calcium chloride dihydrate.
Solvent: water for injections.
What Willfact looks like and contents of the pack
Willfact is presented as powder and solvent for solution for injection after reconstitution with a transfer system.
Willfact is available in pack sizes of 500 IU/5 ml, 1000 IU/10 ml and 2000 IU/20 ml. Marketing Authorisation Holder and Manufacturer
LFB-BIOMEDICAMENTS
3, avenue des Tropiques,
BP 40305-LESULIS,
91958 Courtabceuf Cedex FRANCE
To report a suspected side effect that has not been reported via the Yellow Card Scheme (above), please contact: Medical Information, Wainwright Associates Limited, Wessex House, Marlow Road, Bourne End, Buckinghamshire, SL8 5SP Tel: 01628 531171 - medinfo@wainwrightassociates.co.uk
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria Willfact 500 I.E /1000 I.E /2000 I.E Pulver und Losungsmittel
zur Herstellung einer Injektionslosung Czech Republic Willfact Denmark Willfact
Estonia Willfact
Germany WILLFACT 500 I.E / 1000 I.E / 2000 I.E
Hungary Willfact 500 NE/5 ml; Willfact 1000 NE/10 ml; Willfact 2000
NE/20 ml, por es oldoszer oldatos injekciohoz Latvia Willfact
Lithuania Willfact 500 TV / 1000 TV / 2000TV, milteliai ir tirpiklis
injekciniam tirpalui Norway Willfact
Poland Willfact
Portugal Willfact
Slovenia Willefact 500 i.e 71000 i.e / 2000 i.e Prasek in vehikel za
raztopino za injiciranje Willfact Willfact Willfact Willfact
5033830- 15G077/2.0


o
• Bring the two vials (powder and solvent) to a temperature not above 25°C.
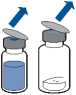
• Remove the protective cap from the solvent vial (water for injections) and from the powder vial.
• Disinfect the surface of each stopper.
• Hold the vial of reconstituted product in vertical position while screwing a sterile syringe onto the Mix2Vial device. Then slowly draw the product up into the syringe.
• Once the product has been transferred to the syringe, firmly hold the syringe (with the piston pointing downward), unscrew the Mix2Vial device and replace it with an intravenous or butterfly needle.
• Expel the air from the syringe and insert into the vein after disinfecting the surface.
• Inject slowly by intravenous route immediately after reconstitution as a single dose at a maximum rate of 4 ml/minute.

The following information is intended for medical or healthcare professionals only:
Reconstitution:
The currently applicable guidelines for aseptic procedures must be followed. The transfer system is only used to reconstitute the drug, as described below. It is not intended in administering the drug to the patient.
Administration:
Any unused product or waste material should be disposed of in accordance with local requirements.
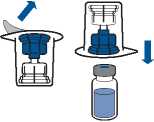
• Remove the cap from the Mix2Vial device. Without removing the device from its packaging, attach the blue end of the Mix2Vial to the stopper of the solvent vial.
• Remove and discard the packaging. Take care not to touch the newly-exposed part of the device.
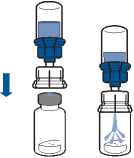
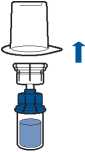
• Turn the solvent vial-device assembly over and attach to the powder vial using the transparent part of the device. The solvent will automatically transfer to the powder vial. Hold the assembly and gently swirl to completely dissolve the product.


• Now, holding the reconstituted product part in one hand and the solvent part in the other, unscrew the Mix2Vial device to separate the vials.
The powder generally dissolves instantaneously and should have dissolved in less than 10 minutes.

The solution should be clear or slightly opalescent, colourless or slightly yellowish. Do not use solutions that are cloudy or have deposits.
WILLFACT
500 IU 1000 IU 2000 IU
Human von Willebrand factor
Powder and solvent for solution for injection package leaflet: information for the user
Read all of this leaflet carefully before you start using this medicine because It contains Important Information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effect not listed in this leaflet. See section 4.
In this leaflet:
1. What Willfact is and what it is used for
2. What you need to know before you use Willfact
3. Howto use Willfact
4. Possible side effects
5. How to store Willfact
6. Contents of the pack and other information
1. WHAT WILLFACT IS AND WHAT IT IS USED FOR
Willfact is a medicine used to stop bleeding that contains human von Willebrand factor (VWF) as active ingredient.
Willfact is indicated in the prevention and treatment of surgical or other bleeding in patients with von Willebrand disease when desmopressin (DDAVP) treatment alone is ineffective or contra-indicated.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE WILLFACT
Contraindications Do not use Willfact
■ if you are allergic to human von Willebrand factor or to any of the other ingredients of this medicine (listed in section 6).
■ if you suffer from haemophilia A.
Warnings and precautions:
Your treatment with Willfact should always be supervised by a physician experienced in the treatment of haemostatic disorders.
If you experience heavy bleeding and a blood examination shows that your Factor VIII blood value is reduced, you will receive the VWF preparation in addition to a Factor VIII preparation within the first twelve hours.
Allergic reactions
As with every protein medicine for intravenous use derived from human blood or plasma, hypersensitivity reactions in the form of an allergy may occur. During your injection, you will be observed specifically to determine whether you experience any early signs of hypersensitivity, e.g. stinging, hives (generalised urticaria), tightness of the chest, wheezing, drop in blood pressure (hypotension) and allergic severe reactions (anaphylaxis). If these symptoms occur, the injection will be interrupted immediately.
Risk of thrombosis
Blood vessels may also become blocked by blood clots (thromboses). This risk exists particularly if your previous medical history or laboratory results indicate that you present certain risk factors. In this case you will be monitored very carefully for the early signs of thrombosis, and a preventative treatment (prophylaxis) against vein blockages by blood clots should be introduced.
When using a Factor VII l-containing VWF product, your physician should be aware that the treatment may cause an excessive rise in FVIIkC. If you receive such FVIII-containing VWF product, your physician should monitor your FVIIkC plasma level regularly. This ensures that your FVIIkC plasma level is not sustained excessive, which may increase the risk of thrombotic events.
Limited effectiveness
It is possible that, in patients with von Willebrand disease, especially type 3 patients, proteins may be formed that neutralise the effect of VWF. These proteins are called antibodies or inhibitors. If the laboratory results give corresponding indications, or if the bleeding does not stop despite a sufficient dose of Willfact, your physician will check whether VWF inhibitors are being formed in your body. If these inhibitors are present in high concentration, treatment with VWF may not be effective, and other treatment options should be considered. The new treatment will be provided by a physician who has experience in the treatment of haemostatic disorders.
Virus safety
When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include:
- careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded,
- the testing of each donation and pools of plasma for signs of virus/infections,
- the inclusion of steps in the processing of the blood or plasma that can inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections. The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus. The measures taken may be of limited value against non-enveloped viruses such as hepatitis A Virus and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals whose immune system is depressed or who have some types of anaemia (e.g. sickle cell disease or haemolytic anaemia).
Vaccinations
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly/repeatedly receive human plasma-derived von Willebrand factor.
Recording of batch number
It is strongly recommended that every time you receive a dose of Willfact the name and batch number of the medicine are recorded in order to maintain a record of the batches used. A