Xalacom Eye Drops
Variation 2 - Only manufacturers details checked. AsifChohan, 3 August 2007
Xalacom® Eye Drops
Patient Information Leaflet
Please read this leaflet carefully before you start using Xalacom. It provides some useful information on your medicine. This medicine has been prescribed for you personally and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours. For further information or advice ask your doctor or pharmacist.
What does Xalacom contain?
Each millilitre (1ml) of Xalacom contains:
Active substances: 50 micrograms latanoprost and timolol maleate corresponding to 5mg timolol.
Other ingredients: benzalkonium chloride 0.2mg/ml (preservative), sodium chloride, sodium dihydrogen phosphate monohydrate, disodium phosphate anhydrous, and water for injections. To bring the solution to the correct pH level, very small amounts of hydrochloric acid or sodium hydroxide may be added to the solution during manufacture.
Each bottle of Xalacom contains 2.5ml eye drops.
What is Xalacom?
Xalacom is a combination of two different medicines that both contribute to lowering the pressure in the eye, but in different ways. Timolol is one of a group of medicines known as “beta-blockers” and lowers the pressure in the eye by reducing the inflow of fluid inside the eye. Latanoprost lowers the pressure by increasing the natural outflow of fluid from the inside of the eye.
Who supplies Xalacom?
This product is manufactured by: Pfizer Manufacturing Belgium NV, Puurs, Belgium.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd, Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: BR Lewis Pharmaceuticals Ltd, Kirk Sandall,
Doncaster, DN3 1QR. _
|POM| PL No: 08929/0128
What is Xalacom used for?
Xalacom is used for lowering increased pressure within the eye (called ocular hypertension) and for the treatment of glaucoma.
When should Xalacom not be used?
Xalacom should not be used if you:
• have a respiratory disease such as asthma or a history of asthma, or have severe respiratory problems due to another cause;
• have heart problems, such as heart rhythm disorders;
• are allergic to any of the ingredients in Xalacom.
If you think any of these points apply to you, do not use Xalacom until you have talked to your doctor.
What else should you know about taking Xalacom?
You should tell your doctor if you have, or have had, asthma or other lung problems, heart problems, circulatory problems, low blood pressure, diabetes or low blood sugar (hypoglycaemia), or allergies to other medicines that you have used.
If you have any heart problems your doctor will want to make sure that the situation is under control before you take your eye drops. Your doctor may want you to have extra checks on your heart and circulation whilst you are using Xalacom.
If you have a coloured part of the eye (iris) that is a mixture of colours, i.e. blue/grey-brown, green-brown or yellow-brown, you may find that you slowly and gradually develop an increase in the brown colour of the iris. Changes in purely blue, grey, green or brown eyes are rare. The brown colour change occurs slowly and is noticed only after months to years. If you are being treated with Xalacom in one eye only, this can result in a difference in colour between eyes. The change in colour is harmless, but will probably be permanent.
If the coloured parts of your eyes are a mixture of colours or if you observe an increase in the brown colour of the iris, talk to your doctor.
Before surgery and anaesthesia, the doctor/dentist should know that you are being treated with Xalacom, since there is a risk that blood pressure can suddenly fall in association with anaesthesia.
The preservative benzalkonium chloride can cause discolouration of soft contact lenses. If you wear contact lenses it is important that the lenses are taken out before applying Xalacom and not replaced until at least 15 minutes after application.
Xalacom is not recommended for children or adolescents.
Pregnancy
Tell your doctor if you are pregnant or planning to become pregnant. Xalacom should not be used during pregnancy.
Breast-feeding
Xalacom should not be used if you are breast-feeding.
Driving and using machines
Possible side effects, e.g. blurred vision, can affect the ability to drive and/or operate machines. If you experience any problems, talk to your doctor.
with other medicines?
Your treatment can be affected if Xalacom is used at the same time as some other medicines. Tell your doctor if you are using or intend to use other medicines, including other eye drops (this also applies to medicines obtained without a prescription). It is important that your doctor knows whether you are already being treated with medication to lower blood pressure, heart medicine, medicine to treat diabetes, medicine to enable you to urinate more easily or in order to restore normal bowel movements, medicine to reduce swelling of the mucous membranes (for nasal congestion) and medicines for asthma that contain adrenaline.
How to use Xalacom
If this is the first time that you have used this bottle, write the date in the space provided on the carton so that you will know when they should no longer be used because they are out of date.
The usual dosage is: 1 drop into the affected eye (eyes) once daily in the morning.
If Xalacom is being used together with other eye drops, they should be put in at least 5 minutes apart.
If a dose is missed, you should wait until the next dose. Do not make up for a missed dose by putting an extra drop into the eye.
Instructions for use
Wash your hands before using your eyedrops.
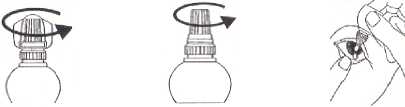
1. Twist off the 2. Unscrew the 3. Using your forefinger,
colourless protective cap. gently pull down the lower
cap with the “wings”. eyelid of the eye that is to
be treated.
4. Place the tip close to the eye and squeeze gently, so that one drop falls into the eye.
5. Replace the cap after use.
If you should accidentally use more Xalacom than you should, always contact your doctor or hospital.
Possible side effects of Xalacom
Like all medicines, Xalacom can cause unwanted effects, known as side effects.
A change in eye colour was seen in 16-20% of all patients after a year. This was more frequent in patients in whom the coloured part of the eye (iris) was mixed, e.g. blue/grey-brown, green-brown or yellow-brown. The iris may become darker and more brown in colour. This difference in eye colour can also be noticeable if only one eye is being treated. In patients with single coloured eyes, e.g. blue, grey, green or brown, however, noticeable changes in eye colour are rare.
Darker, thicker and longer eyelashes were seen in 37% of patients. Irritation, including stinging, burning and itching, slight pain in the eye (eyes), redness of the white of the eye and/or edges of the eyelid, superficial changes to the cornea and blurred vision, skin rash and headache were also seen.
The following side-effects have been seen with latanoprost or timolol and so may possibly be seen with Xalacom. Visual disturbances (such as double vision), swelling of the yellow spot, conjunctival catarrh and drooping eyelid. Dark colour of the skin of the eyelid. Inflammation and swelling in and around the eye/eyelid. Inflammation of the cornea and inflammation of the iris. Asthma, acute and worsened asthma, laboured breathing, shortness of breath.
When the solution is instilled into the eye, a small amount of active substance can be absorbed into the blood and cause effects elsewhere in the body, not only in the eye. These include fatigue (tiredness), dizziness, light-headedness, nausea, depression, low blood pressure, chest pain and effects on the heart rhythm, heart failure. Nightmares and reduced sexual urge. Hypersensitivity reactions such as skin rash, nettle rash, hair loss and a worsening of myasthenia gravis (increased muscle weakness).
If any disturbing or unusual reactions occur, contact your doctor.
How to store Xalacom
Keep your bottle out of the reach and sight of children.
Store at 2°C - 8°C.
Opened container: Do not store above 25°C.
Keep the container in the outer carton.
Xalacom should not be used after the expiry date on the bottle or 4 weeks after opening the container for the first time.
If you eye drops become discoloured or show any other signs of deterioration, return to your pharmacist for advice.
Leaflet revision and issue date: 29.09.05
Xalacom® is a registered trademark of Pharmacia.