Xeomin 50 Ld50 Units Powder For Solution For Injection
Out of date information, search anotherreading direction

reading direction

#
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
1. What XEOMIN is and what it is used for
2. What you need to know before you use XEOMIN
3. How to use XEOMIN
4. Possible side effects
Package leaflet: Information for the user
XEOMIN 0
50 units powder for solution for injection
Clostridium Botulinum neurotoxin type A (150 kD), free from complexing proteins
What is in this leaflet:
1. What XEOMIN is and what it is used for
2. What you need to know before you use XEOMIN
3. How to use XEOMIN
4. Possible side effects
5. How to store XEOMIN
6. Contents of the pack and other information
XEOMIN is a medicine that relaxes the muscles.
XEOMIN is used for the treatment of the following conditions in
adults:
• eyelid spasm (blepharospasm)
• twisted neck (spasmodic torticollis)
• increased muscle tension/uncontrollable muscle stiffness in arms or hands after a stroke (post-stroke spasticity of the upper limb, going along with clinical pattern of flexed wrist and clenched fist)
Do not use XEOMIN
• if you are allergic to Botulinum neurotoxin type A or any of the other ingredients of this medicine (listed in section 6)
• if you suffer from a generalised disorder of muscle activity (e.g. myasthenia gravis, Lambert-Eaton syndrome)
• if you have an infection or inflammation at the proposed injection site.
Warnings and precautions
Side effects may occur from misplaced injections of Botulinum neurotoxin type A temporarily paralysing nearby muscle groups. There have been very rare reports of side effects that may be related to the spread of Botulinum toxin far from the injection site (e.g. excessive muscle weakness, swallowing difficulties or accidental swallowing of food or drink into the airways). Patients who receive the recommended doses may experience excessive muscle weakness.
If the dose is too high or the injections too frequent, the risk of antibody formation may increase. Antibody formation can cause treatment with Botulinum toxin type A to fail, whatever the reason for its use.
Talk to your doctor before XEOMIN is used:
• if you suffer from any type of bleeding disorder
• if you receive substances that prevent the blood from clotting (e.g. coumarin, heparin, acetylsalicylic acid, clopidogrel)
• if you suffer from pronounced weakness or decreased muscle volume in the muscle where you will receive the injection
• if you suffer from amyotrophic lateral sclerosis (ALS), which can lead to generalised muscle decrease.
• if you suffer from any disease that disturbs the interaction between nerves and skeletal muscles (peripheral neuromuscular dysfunction)
• if you have or have had swallowing difficulties
• if you have had problems with injections of Botulinum toxin type A in the past
• if you are due to have surgery MN xxxxx, GB/IE
Repeated injections with XEOMIN
If you have repeated injections with XEOMIN, the effect may increase or decrease. Possible reasons for this are:
• your doctor may follow a different procedure when preparing the solution for injection
• different treatment intervals
• injections into another muscle
• marginally varying effectiveness of the active substance of XEOMIN
• non-response/therapy failure during the course of treatment
If you have been inactive for a long period of time, any activity should be started gradually after the XEOMIN injection.
Contact your doctor and seek medical attention immediately if you experience any of the following:
• difficulty in breathing, swallowing or speaking
• hives, swelling including swelling of the face or throat, wheezing, feeling faint and shortness of breath (possible symptoms of severe allergic reactions)
Eyelid spasm (blepharospasm)
Talk to your doctor before XEOMIN is used, if you:
• have had an eye surgery. Your doctor will then take additional precautions.
• are at risk of developing a disease called narrow angle glaucoma. This disease can cause the inner eye pressure to rise and may lead to a damaging of your optic nerve. Your doctor will know if you are at risk.
During treatment, small punctuated bleedings may occur in the soft tissues of the eyelid. Your doctor can limit these by immediately applying gentle pressure at the injection site.
After you receive a XEOMIN injection into your eye muscle your blinking rate may be reduced. This can lead to a prolonged exposure of the transparent front part of the eye (cornea). This exposure may lead to a damaging of the surface and an inflammation (corneal ulceration).
Twisted neck (spasmodic torticollis)
After the injection you may develop mild to severe swallowing difficulties. This may lead to problems with breathing and you may have a higher risk of inhaling foreign substances or fluids. Foreign substances in your lungs may lead to an inflammation or infection (pneumonia). Your doctor will give you special medical treatment if needed (e.g. in the form of artificial nutrition).
Swallowing difficulties can last for up to two to three weeks after injection, for one patient a duration of up to five months is known.
Increased muscle tension/uncontrollable muscle stiffness in arms or hands after a stroke (post-stroke spasticity of the upper limb)
XEOMIN can be used to treat increased muscle tension/uncontrollable muscle stiffness in parts of your upper limb, e.g. your elbow, forearm or hand. XEOMIN is effective in combination with the usual standard treatment methods. XEOMIN should be used together with these other methods.
It is unlikely that this medicine will improve the range of motion of joints where the surrounding muscle has lost its ability to stretch.
Children and adolescents
Do not give this medicine to children between the ages of 0-17 years because the use of XEOMIN in children and adolescents has not yet been investigated and is therefore not recommended.
Other medicines and XEOMIN
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The effect of XEOMIN may be increased by:
• medicines used to treat certain infectious diseases (specti-nomycin or aminoglycoside antibiotics [e.g. neomycin, ka-namycin, tobramycin])
• other medicines that relax the muscles (e.g. muscle rela-xants of the tubocurarine-type). Such medicines are used, for example, in general anaesthesia. Before you have surgery, tell your anaesthetist if you have received XEOMIN.
In these cases, XEOMIN must be used carefully.
The effect of XEOMIN may be reduced by certain medicines for malaria and rheumatism (known as aminoquinolines).
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before this medicine is administered.
XEOMIN should not be used during pregnancy, unless your doctor decides that the necessity and potential benefit of the treatment justifies the possible risk on the foetus.
XEOMIN is not recommended if you are breast-feeding.
Driving and using machines
You should not drive or engage in other potentially hazardous activities if drooping eyelids, weakness (asthenia), muscle weakness, dizziness or vision disorder occur.
If in doubt ask your doctor for advice.
XEOMIN may only be administered by doctors with appropriate specialist knowledge of treatment with Botulinum neurotoxin.
The optimum dosage and number of injection sites in the treated muscle will be chosen by your doctor individually for you. The results of initial treatment with XEOMIN should be evaluated and may lead to dose adjustment until the desired therapeutic effect is achieved.
If you have the impression that the effect of XEOMIN is too strong or too weak, let your doctor know. In cases where no therapeutic effect is apparent, alternative therapies should be taken into consideration.
Eyelid spasm (blepharospasm)
Usually, the first onset of effect is observed within four days after injection. The effect of each treatment generally lasts for about 3-4 months, however, it may last significantly longer or shorter. The treatment can be repeated if required. The recommended initial dose is up to 25 units per eye, and the total recommended dose in follow-up treatment sessions is up to 100 units per session.
Normally, no additional benefit is conferred by treating more frequently than every three months.
Twisted neck (spasmodic torticollis)
Usually, the first onset of effect is observed within seven days after injection. The effect of each treatment generally lasts for about 3-4 months, however, it may last significantly longer or shorter. Treatment intervals of less than 10 weeks are not recommended. The recommended dose per single injection site is up to 50 units, and the maximum dose for the first treatment session is 200 units. Doses up to 300 units may be given by your doctor in subsequent courses depending on the response.
Increased muscle tension/uncontrollable muscle stiffness in arms or hands after a stroke (post-stroke spasticity of the upper limb)
Patients reported the onset of action 4 days after treatment. An improvement of muscle tone was perceived within 4 weeks. In general, the treatment effect lasted 12 weeks. The recommended dose is up to 400 units per treatment session. The period between each treatment session should be at least 12 weeks.
Dissolved XEOMIN is intended for injections into the muscle.
If you are given more XEOMIN than you require
Symptoms of overdose:
Symptoms of overdose are not apparent immediately after the injection and may include general weakness, drooping eyelid, double vision, breathing difficulties, speech difficulties, and paralysis of the respiratory muscles or swallowing difficulties which may result in pneumonia.
Measures in cases of overdose:
In case you feel symptoms of overdose please seek medical emergency services immediately or ask your relatives to do so, and have yourself admitted to hospital. Medical supervision for up to several days and assisted ventilation may be necessary.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects such as excessive muscle weakness or swallowing difficulties are caused by the relaxation of muscles far from the injection site of XEOMIN. Swallowing difficulties can cause inhalation of foreign bodies resulting in lung inflammation and in some cases, death.
An allergic reaction may occur with XEOMIN. Serious immediate allergic reactions (anaphylaxis) or allergic reactions to the serum in the product (serum sickness), causing for example difficulty in breathing (dyspnoea), hives (urticaria) or swelling of the soft tissue (oedema), have been rarely reported. Some of these reactions have been observed following the use of conventional Botulinum toxin type A complex. They occurred when the toxin was given alone or in combination with other products known to cause similar reactions. An allergic reaction can cause any of the following symptoms:
• difficulty with breathing, swallowing or speaking due to the swelling of the face, lips, mouth or throat
• swelling of the hands, feet or ankles.
If you notice any of these side effects, please inform your doctor immediately or go to the accident and emergency department of your nearest hospital or ask your relatives to do so.
Usually, side effects are observed within the first week after treatment and are temporary in nature. Side effects may be related to the medicine, injection technique or both. Side effects may be restricted to the area around the injection site (e.g. localised muscle weakness, local pain, inflammation, pins and needles (paraesthesia), reduced sense of touch (hypoaesthe-sia), tenderness, swelling (general), swelling of the soft tissue (oedema), skin redness (erythema), itching, localised infection, haematoma, bleeding and/or bruising).
The injection of the needle may cause pain. This pain or the anxiety towards needles may lead to fainting or a low blood pressure.
0
#
Eyelid spasm (blepharospasm)
The following side effects were reported with XEOMIN:
Very common (may affect more than 1 in 10 people):
Drooping eyelid (ptosis), dry eyes
Common (may affect up to 1 in 10 people):
Vision blurred, visual impairment, double vision (diplopia), lac-rimation increased, dry mouth, swallowing difficulties (dysphagia), headache, injection site pain, fatigue, muscular weakness, weakness of face muscle (facial paresis), rash
Twisted neck (spasmodic torticollis)
The following side effects were reported with XEOMIN:
Very common (may affect more than 1 in 10 people):
Swallowing difficulties (dysphagia)
Common (may affect up to 1 in 10 people):
Neck pain, muscular weakness, musculoskeletal pain (myalgia), musculoskeletal stiffness, muscle spasm, headache, dizziness, injection site pain, weakness (asthenia), dry mouth, nausea, sweating increased (hyperhidrosis), upper respiratory tract infection, feeling faint (presyncope)
Uncommon (may affect up to 1 in 100 people):
Speech disorders (dysphonia), shortness of breath (dyspnoea), rash
The treatment of twisted neck may cause swallowing difficulties with varying degrees of severity. This may lead to breathing in foreign materials, which may require medical intervention. Swallowing difficulties may persist for two to three weeks after injection, but has been reported in one case to last five months. Swallowing difficulties appear to be dose-dependent.
Increased muscle tension/uncontrollable muscle stiffness in arms or hands after a stroke (post-stroke spasticity of the upper limb)
The following side effects were reported with XEOMIN:
Common (may affect up to 1 in 10 people):
Headache, decreased or abnormal skin sensation including partial loss of sensation or sensation of heat (dysesthesia, hypoes-thesia), muscular weakness, feeling hot, injection site pain, pain in extremity, swallowing difficulties (dysphagia)
Uncommon (may affect up to 1 in 100 people):
Weakness (asthenia), myalgia
Some of these side effects may be disease related.
Post-marketing experience
There have been reports of flu-like symptoms and hypersensitivity reactions, such as swelling, swelling of the soft tissue (oedema), redness, itching, rash (local and generalised) and breathlessness.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard (UK) or via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@hpra.ie (IE). By reporting side effects, you can help provide more information on the safety of this medicine.
Pack sizes of 1,2, 3 or 6 vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Merz Pharmaceuticals GmbH Eckenheimer LandstraBe 100 60318 Frankfurt/Main Germany
Manufacturer
Merz Pharma GmbH & Co. KGaA Eckenheimer LandstraBe 100 60318 Frankfurt/Main PO. Box 11 13 53 60048 Frankfurt/Main Germany
Telephone: +49-69/15 03-1 Fax: +49-69/15 03-200
This medicinal product is authorised in the Member States of the EEA under the following names:
XEOMIN: Austria, Bulgaria, Cypress, Croatia, Czech
Republic, Denmark, Estonia, Germany, Greece, Finland, France, Hungary, Ireland, Island, Italy, Latvia, Lichtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom
XEOMEEN: Belgium
This leaflet was last revised in 03/2015.
After inactivation used vials, syringes and materials should not be emptied and must be discarded into appropriate containers and disposed of in accordance with local requirements.
Recommendations should any incident occur during the handling of Botulinum toxin
• Any spills of the product must be wiped up: either using absorbent material impregnated with any of the above solutions in case of the powder, or with dry, absorbent material in case of reconstituted product.
• The contaminated surfaces should be cleaned using absorbent material impregnated with any of the above solutions, then dried.
• If a vial is broken, proceed as mentioned above by carefully collecting the pieces of broken glass and wiping up the product, avoiding any cuts to the skin.
• If the product comes into contact with skin, rinse the affected area abundantly with water.
• If product gets into the eyes, rinse thoroughly with plenty of water or with an ophthalmic eyewash solution.
• If product comes into contact with a wound, cut or broken skin, rinse thoroughly with plenty of water and take the appropriate medical steps according to the dose injected.
These instructions for use, handling and disposal should be strictly followed.
The following information is intended for medical or healthcare professionals only:
Instructions for reconstitution of the solution for injection:
XEOMIN is reconstituted prior to use with sodium chloride 9 mg/ml (0.9 %) solution for injection.
XEOMIN may only be applied for its intended use to treat one patient for one session.
It is good practice to reconstitute the vial contents and prepare the syringe over plastic-lined paper towels to catch any spillage. An appropriate amount of solvent (see dilution table) is drawn up into a syringe. After vertical insertion of the needle through the rubber stopper, the solvent is injected gently into the vial in order to avoid foam formation. A 20-27 G short bevel needle is recommended for reconstitution. The vial must be discarded if the vacuum does not pull the solvent into the vial. Remove the syringe from the vial and mix XEOMIN with the solvent by carefully swirling and inverting the vial - do not shake vigorously. If needed, the needle used for reconstitution should remain in the vial and the required amount of solution should be drawn up with a new sterile syringe suitable for injection.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial label after “EXP”. The expiry date refers to the last day of that month.
Unopened vial: Do not store above 25 °C.
Reconstituted solution: Chemical and physical in-use stability has been demonstrated for 24 hours at 2 °C to 8 °C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 °C to 8 °C, unless reconstitution has taken place in controlled and validated aseptic conditions.
Your doctor should not use XEOMIN if the solution has a cloudy appearance or contains visible particles.
For instructions on disposal, please see information for medical and healthcare professionals at the end of this leaflet.
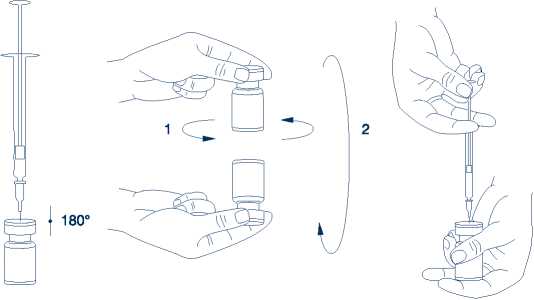
Reconstituted XEOMIN is a clear, colourless solution free of particulate matter.
XEOMIN must not be used if the reconstituted solution (prepared as above) has a cloudy appearance or contains floccular or particulate matter.
Possible dilutions are indicated in the following table:
|
Solvent added |
Resulting dose in |
|
(sodium chloride 9 mg/ml (0.9 %) solution for injection) |
units per 0.1 ml |
0.25 ml
20.0 U
0.5 ml
10.0 U
1.0 ml
5.0 U
What XEOMIN contains
• The active substance is: Clostridium Botulinum neurotoxin type A (150 kD), free from complexing proteins.
One vial contains 50 LD50 units Clostridium Botulinum neurotoxin type A (150 kD), free from complexing proteins. Due to differences in the LD50 potency assay, these units are specific to XEOMIN and are not applicable to other Botulinum toxin preparations.
• The other ingredients are: human albumin, sucrose.
What XEOMIN looks like and contents of the pack
XEOMIN is presented as a powder for solution for injection. The powder is white.
Dissolving the powder produces a clear, colourless, particle-free solution.
MN xxxxx, GB/IE
2.0 ml
2.5 U
4.0 ml
1.25 U
Instructions for disposal
Any solution for injection that has been stored for more than 24 hours as well as any unused solution for injection should be discarded.
Procedure to follow for a safe disposal of vials, syringes and materials used
Any unused vials, residual reconstituted solution in the vial and/ or syringes should be autoclaved. Alternatively, the remaining XEOMIN can be inactivated by adding one of the following solutions: 70 % ethanol, 50 % isopropanol, 0.1 % SDS (anionic detergent), diluted sodium hydroxide solution (0.1 N NaOH), or diluted sodium hypochlorite solution (at least 0.1 % NaOCl).