Zaditen Eye Drops
S0145 LEAFLET Zaditen 20160620
PACKAGE LEAFLET: INFORMATION FOR THE USER ZADITEN® EYE DROPS (ketotifen)
Your medicine is known as Zaditen Eye Drops but will be referred to as Zaditen throughout the following leaflet.
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Zaditen is and what it is used for
2. Before you use Zaditen
3. How to use Zaditen
4. Possible side effects
5. How to store Zaditen
6. Further information
Important information about some of the ingredients of Zaditen
Zaditen contains benzalkonium chloride and may cause eye irritation.
If you wear soft contact lenses you should remove them before using Zaditen as it can discolour your soft contact lenses. You should wait at least 15 minutes after using Zaditen before reinserting your contact lenses into your eyes.
3. HOW TO USE ZADITEN
Always use Zaditen exactly as your doctor has told you. You should
check with your doctor or pharmacist if you are not sure.
The usual dose for adults, elderly and children (age 3 and older) is
one drop into the affected eye(s) twice a day (in the morning and
evening).
Instructions for use
1. Wash your hands.
2. Open the bottle. Do not touch the tip after opening the bottle.
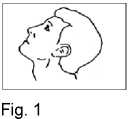
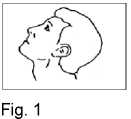
3. Lean your head back (Fig. 1).
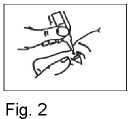
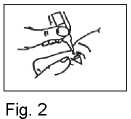
4. Pull down your lower eyelid with your finger and hold the bottle in your other hand. Squeeze the bottle so that one drop falls into the eye (Fig. 2).
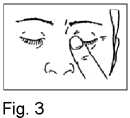
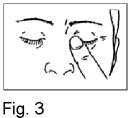
5. Close your eyes and press the tip of one finger against the corner of the eye for around 1-2 minutes. This will prevent the drop running through the tear duct into your throat and most of the drop will remain in the eye (Fig. 3). If necessary repeat steps 3 to 5 with your other eye.
6. Close the bottle after use.
1. WHAT ZADITEN IS AND WHAT IT IS USED FOR
Zaditen contains the active substance ketotifen, which is an antiallergic substance. Zaditen is used to treat eye symptoms of hay fever.
2. BEFORE YOU USE ZADITEN
Do not use Zaditen
If you are allergic (hypersensitive) to ketotifen or any of the other ingredients of Zaditen.
Using other medicines
If you need to apply any other medicinal products to your eyes together with Zaditen, wait at least 5 minutes between applying each product.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. This is particularly important for medicines which are used to treat:
• depression
• allergy (e.g. antihistamines)
Using Zaditen with food and drink
Zaditen may increase the effect of alcohol.
Pregnancy and breast-feeding
If you are pregnant or think you might be, ask your doctor or pharmacist for advice before using Zaditen.
Zaditen can be used during breast-feeding.
Driving and using machines
Zaditen may cause blurred vision or drowsiness. If this happens to you, wait until this has cleared before driving or operating machinery.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you use more Zaditen than you should
There is no danger if you have accidentally taken Zaditen by mouth or if you have used more than one drop in the eye. If you have any doubt contact your doctor for advice.
If you forget to use Zaditen
If you forget to use Zaditen you should treat your eyes as soon as you remember.
Then continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Zaditen can cause side effects, although not everybody gets them.
The following side effects have been reported.
Common (affecting less than 1 in every 10 patients)
• eye irritation or pain
• inflammation in the eye
• eye pain, blurred vision, abnormal intolerance to light
Uncommon (affecting less than 1 in every 100 patients)
• blurred vision when putting drops on the eye
• dry eye
• eyelid disorder
• conjunctivitis
• increased sensitivity of the eyes to light
• visible bleeding in white of eye
• headache
• drowsiness
• rash (which may also itch)
• eczema (itchy, red, burning rash)
• dry mouth
• allergic reaction (including swelling of the face and eyelids) and increase in severity of existing allergic conditions such as asthma and eczema
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE ZADITEN
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store Zaditen above 25°C. Discard any unused solution 28 days after first opening.
• Do not use after the expiry date printed on the carton or bottle label.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Zaditen contains
• Each ml of solution contains ketotifen hydrogen fumarate equivalent to 0.25mg ketotifen as the active ingredient.
• Zaditen also contains the following: benzalkonium chloride, glycerol, sodium hydroxide and water for injection. These are the inactive ingredients.
What Zaditen looks like and contents of the pack
• Zaditen is a colourless solution.
• Zaditen is available in 5ml bottle.
Product Licence holder
Procured from within the EU and repackaged by the parallel import product licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HA0 1DX.
Manufacturer
This product is manufactured by either:
• EXCELVISION, rue de la Lombardiere, 07100 Annonay, France or
• Novartis Pharma GmbH, Vienna, Austria.
|POM | PL: 19488/0145
Leaflet revision date: 20 June 2016
Zaditen is a registered trade mark of Novartis AG, Switzerland.
S0145 LEAFLET Zaditen 20160620
S0145 LEAFLET Ketotifen 20160620
PACKAGE LEAFLET: INFORMATION FOR THE USER KETOTIFEN 0.25mg/ml EYE DROPS (ketotifen)
Your medicine is known as Ketotifen 0.25mg/ml Eye Drops but will be referred to as Ketotifen throughout the following leaflet.
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Ketotifen is and what it is used for
2. Before you use Ketotifen
3. How to use Ketotifen
4. Possible side effects
5. How to store Ketotifen
6. Further information
1. WHAT KETOTIFEN IS AND WHAT IT IS USED FOR
Ketotifen contains the active substance ketotifen, which is an antiallergic substance. Ketotifen is used to treat eye symptoms of hay fever.
2. BEFORE YOU USE KETOTIFEN
Do not use Ketotifen
If you are allergic (hypersensitive) to ketotifen or any of the other ingredients of Ketotifen.
Using other medicines
If you need to apply any other medicinal products to your eyes together with Ketotifen, wait at least 5 minutes between applying each product.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. This is particularly important for medicines which are used to treat:
• depression
• allergy (e.g. antihistamines)
Using Ketotifen with food and drink
Ketotifen may increase the effect of alcohol.
Pregnancy and breast-feeding
If you are pregnant or think you might be, ask your doctor or pharmacist for advice before using Ketotifen.
Ketotifen can be used during breast-feeding.
Driving and using machines
Ketotifen may cause blurred vision or drowsiness. If this happens to you, wait until this has cleared before driving or operating machinery.
Important information about some of the ingredients of Ketotifen
Ketotifen contains benzalkonium chloride and may cause eye irritation.
If you wear soft contact lenses you should remove them before using Ketotifen as it can discolour your soft contact lenses. You should wait at least 15 minutes after using Ketotifen before reinserting your contact lenses into your eyes.
3. HOW TO USE KETOTIFEN
Always use Ketotifen exactly as your doctor has told you. You
should check with your doctor or pharmacist if you are not sure.
The usual dose for adults, elderly and children (age 3 and older) is
one drop into the affected eye(s) twice a day (in the morning and
evening).
Instructions for use
1. Wash your hands.
2. Open the bottle. Do not touch the tip after opening the bottle.
3. Lean your head back (Fig. 1).
4. Pull down your lower eyelid with your finger and hold the bottle in your other hand. Squeeze the bottle so that one drop falls into the eye (Fig. 2).
5. Close your eyes and press the tip of one finger against the corner of the eye for around 1-2 minutes. This will prevent the drop running through the tear duct into your throat and most of the drop will remain in the eye (Fig. 3). If necessary repeat steps 3 to 5 with your other eye.
6. Close the bottle after use.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you use more Ketotifen than you should
There is no danger if you have accidentally taken Ketotifen by mouth or if you have used more than one drop in the eye. If you have any doubt contact your doctor for advice.
If you forget to use Ketotifen
If you forget to use Ketotifen you should treat your eyes as soon as you remember.
Then continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Ketotifen can cause side effects, although not everybody gets them.
The following side effects have been reported.
Common (affecting less than 1 in every 10 patients)
• eye irritation or pain
• inflammation in the eye
• eye pain, blurred vision, abnormal intolerance to light
Uncommon (affecting less than 1 in every 100 patients)
• blurred vision when putting drops on the eye
• dry eye
• eyelid disorder
• conjunctivitis
• increased sensitivity of the eyes to light
• visible bleeding in white of eye
• headache
• drowsiness
• rash (which may also itch)
• eczema (itchy, red, burning rash)
• dry mouth
• allergic reaction (including swelling of the face and eyelids) and increase in severity of existing allergic conditions such as asthma and eczema
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE KETOTIFEN
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store Ketotifen above 25°C. Discard any unused solution 28 days after first opening.
• Do not use after the expiry date printed on the carton or bottle label.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Ketotifen contains
• Each ml of solution contains ketotifen hydrogen fumarate equivalent to 0.25mg ketotifen as the active ingredient.
• Ketotifen also contains the following: benzalkonium chloride, glycerol, sodium hydroxide and water for injection. These are the inactive ingredients.
What Ketotifen looks like and contents of the pack
• Ketotifen is a colourless solution.
• Ketotifen is available in 5ml bottle.
Product Licence holder
Procured from within the EU and repackaged by the parallel import product licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HA0 1DX.
Manufacturer
This product is manufactured by either:
• EXCELVISION, rue de la Lombardiere, 07100 Annonay, France or
• Novartis Pharma GmbH, Vienna, Austria.
|POM | PL: 19488/0145
Leaflet revision date: 20 June 2016
S0145 LEAFLET Ketotifen 20160620