Zerseos 0.5 Mg/2.5 Mg Per 2.5 Ml Nebuliser Solution
What is in this leaflet:
1. What Zerseos is and what it is used for
2. What you need to know before you use Zerseos
3. How to use Zerseos
4. Possible side effects
5. How to store Zerseos
6. Contents of the pack and other information
1. What Zerseos is and what it is used for
3. How to use Zerseos
C
A
B
Package leaflet: Information for the user
Zerseos 0.5 mg/2.5 mg nebuliser solution
Ipratropium bromide and salbutamol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Zerseos. The active ingredients are ipratropium bromide and salbutamol.
Ipratropium bromide and salbutamol both belong to a group of medicines called bronchodilators, which help to improve your breathing by opening up your airways. This is achieved by preventing the contraction of the smooth muscles surrounding the airways, therefore allowing the airways to remain open. Ipratropium bromide acts by blocking the nerve signals that go to the muscles surrounding the airways, and salbutamol acts by stimulating the beta2 receptors in the muscles.
Zerseos is used to treat breathing problems in people 12 years of age and older with long- standing breathing difficulties (chronic obstructive pulmonary disease such as chronic bronchitis, emphysema). Zerseos will relieve wheezing, shortness of breath and chest tightness.
You use it with a device called a ‘nebuliser’. This changes your medicine into a mist for you to breathe in.
2. What you need to know before you use Zerseos
Do not use Zerseos:
• if you know that you have an enlarged heart or a condition known as hypertrophic obstructive cardiomyopathy or HOCM
• if you suffer from fast heart rhythms
• if you are allergic to salbutamol, ipratropium bromide, atropine (including drugs similar to atropine) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before using Zerseos:
• if you suffer or think you may suffer from an eye condition known as glaucoma (increased pressure in the eyes) or if you suffer from any other eye conditions. Your doctor may advise you to protect your eyes when using Zerseos
• if you know that you (if male) have an enlarged prostate or if you have problems passing urine (water)
• if you have had a recent heart attack (myocardial infarction)
• if you have problems with your arteries or get pain in your legs when you walk
• if you have a history of heart disease, irregular heart rhythm or angina (please tell your doctor before starting this medication)
• if you have diabetes
• if you have an overactive thyroid gland
• if you suffer from cystic fibrosis
• if you have been told that you have a tumour of the adrenal gland
Dental caries have been reported with salbutamol use. It is recommended, particularly in children, to pay attention to proper oral hygiene and perform regular dental checkups.
Consult your doctor in case of sudden worsening of your breathing disorders or when the prescribed dose does not give a normal result. Do not increase the dose without doctor’s advice.
If you use high doses of Zerseos for a long time, the amount of potassium in your blood must be monitored, especially if you are taking certain other medicines at the same time such as: steroids (corticosteroids), drugs that increase the production of urine (diuretics) or other drugs that open up the airways such as theophylline (xanthines).
Children and adolescents
Zerseos Nebuliser solution should not be used in children under 12 years of age.
Other medicines and Zerseos
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Certain medicines may interact with Zerseos and may worsen side effects or reduce the effect of Zerseos. You must always tell your doctor if you are taking any of the following medicines:
• other medicines that help your breathing, such as salbutamol and “preventers” such as beclometasone dipropionate. These may increase the effect of Zerseos and increase the severity of side effects.
• beta-blockers i.e. medicines that are commonly used to treat heart conditions such as chest pain that occurs on exertion (called angina pectoris), irregular heart beats or arrhythmias and high blood pressure (called hypertension). They include medicines, such as propranolol, which may cause blood potassium levels to fall when given at the same time as Zerseos (beta-blockers can reduce the effect of salbutamol)
• certain medicines to treat depression (“anti-depressants). This class of medicines includes monoamine oxidase inhibitors (e.g. phenelzine) or tricyclic antidepressants (e.g. amitriptyline).
Page 1
• digoxin (for heart problems) may cause heart rhythm problems when given with Zerseos
• medicines called ‘anti-cholinergics’. These can be used to treat colic pain, Parkinson’s disease, problems passing water or lack of control of your bladder or bowels.
• a reduction in the level of potassium (hypokalaemia) in the blood due to the salbutamol component of Zerseos can occur more likely if you are taking Zerseos with some other asthma treatments, with inhaled steroids or steroid tablets or with diuretics (“water tablets”). A low level of potassium in the blood may cause muscle weakness, twitching or abnormal heart rhythm. Your doctor may need to take a blood test to measure your potassium levels from time to time.
• anaesthetic agents may increase the susceptibility of the effects of salbutamol on the heart - you will be monitored closely or your doctor might decide to discontinue Zerseos if you are going to have an operation.
If you are going to have a general anaesthetic in hospital, please tell the anaesthetist what drugs you are taking.
Zerseos with food and drink
Food and drink have no influence on Zerseos.
Pregnancy and breast-feeding
If you are pregnant or think you may be pregnant or are planning to have a baby, ask your doctor, pharmacist or nurse for advice before taking this medicine.
Do not use Zerseos if you are pregnant unless your doctor decides that the benefit to you outweighs any risk to your child.
Zerseos can be used during breast feeding. Ask your doctor, pharmacist or nurse for advice before taking this medicine during breast feeding.
Driving and using machines
If you experience side effects such as dizziness, difficulty focusing and blurred vision during treatment with Zerseos you should avoid potentially dangerous tasks such as driving or operating machinery.
Zerseos is for inhalation use. The nebuliser solution is for oral inhalation after nebulisation.
Always use this medicine exactly as your doctor has told you. Check with your doctor or nurse if you are not sure.
The recommended dose for adults and children over 12 years of age is 1 ampoule, three or four times a day.
Elderly patients should take the usual adult dose.
Use in children
Zerseos is not recommended for children under 12 years of age.
The label will tell you how much to take and how often to take it.
Never use more medicine than your doctor has told you to. Tell your doctor if your breathing problems get worse or your medicine does not provide as much relief from your breathing problems as before or if you are using your blue short-acting “reliever” inhaler more often than is usual for you.
Zerseos should be used with a suitable nebuliser, e.g. PARI LC PLUS Nebuliser, jet nebulizer. Please read the full instructions for use of the nebuliser in the leaflet provided with PARI LC PLUS before starting the inhalation.
Instructions for use
• Prepare your nebuliser for use according to the manufacturer’s instructions and advice from your doctor.
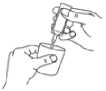
Carefully remove an ampoule from the labelled strip by twisting and pulling. Never use an ampoule that has been opened already or if the nebuliser solution is discoloured (diagram A).
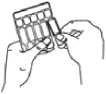
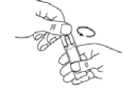
Hold the ampoule upright and twist off the cap (diagram B).
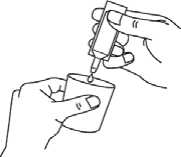
Squeeze the contents into the reservoir of your nebuliser (diagram C).
Follow the manufacturer’s instructions and the advice from your doctor on how to assemble and how to use your nebuliser.
After you have used your nebuliser, throw away any nebuliser solution that is left in the reservoir. Any nebuliser solution left in the ampoule should also be thrown away.
Clean your nebuliser thoroughly according to the manufacturer’s instructions.
Do not dilute the nebuliser solution or mix it with other medicines, unless your doctor tells you to.
The single dose ampoules of Zerseos do not contain preservatives and therefore it is important to use the contents immediately after opening. A new ampoule must be used each time you use Zerseos in your nebuliser.
Partly used, opened or damaged ampoules should be discarded. You should never use an ampoule, which has been opened earlier.
It is important that you follow these instructions in order to avoid any contamination of the nebuliser solution within the ampoules.
Do not swallow the nebuliser solution or use it in injections.
Do not allow the nebuliser solution or mist to enter your eyes. If any of the liquid or mist accidentally gets into your eyes you may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, ask your doctor for advice. If you get problems with your eyes at any other time, ask your doctor for advice.
If you use more Zerseos than you should
If you have taken a slightly larger dose than usual, you may notice a faster heart beat (palpitations) or tremor. Other symptoms might include chest pain, changes in blood pressure, flushing, restlessness or dizziness. These effects usually wear off in a few hours. The level of potassium in your blood may fall and the doctor
Page 2
Zerseos 0.5 mg/0.25 mg nebuliser solution
2D
Code
Area
XXXXX
xxxxx
iM.iy
■’Po.)
az
uopnjos .iasi|iiqau §ui sro/§ui S'O
S03SJ3Z
4. Possible side effects
5. How to store Zerseos
may want to monitor the potassium in your blood by taking a blood test to measure the levels from time to time. Tell your doctor if you are worried by any of these symptoms or if they persist.
If you have taken more medicine than you should, tell your doctor immediately or go to the nearest hospital.
If you need to visit a doctor or need to go into hospital then you should take all of your medicines with you, including any you may have bought without a prescription; these should be in their original packaging if at all possible. Take this leaflet with you to show the doctor.
If you forget to use Zerseos
If you forget to take a dose at the right time, take it as soon as you remember. Do not take a double dose to make up for a forgotten dose.
If you stop using Zerseos
Your doctor will tell you how long you need to use Zerseos . You should not stop using Zerseos without first talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them. Some of the side-effects may be serious and require medical intervention.
Serious side effects
• If your breathing problem or wheezing gets worse immediately after inhaling Zerseos, or your breathing becomes more difficult and you become short of breath do not take any more Zerseos but use your short-acting ‘reliever’ inhaler straightaway. You should stop using Zerseos and should contact your doctor immediately. Your doctor may prescribe alternative treatment for your condition.
• If you think that you may be allergic to Zerseos or if you think you may be having an allergic reaction to the nebuliser solution including swelling, which may effect the tongue, lips and face, then you should stop using Zerseos straightaway and contact your doctor immediately.
Side effects can occur with the following frequencies:
Common, may affect up to 1 in 10 people
• dryness of the mouth
• nausea (feeling sick)
• mouth and throat irritation Uncommon, may affect up to 1 in 100 people
• headaches
• dizziness
• feeling of nervousness
• tremor
• feeling of dizziness or spinning (vertigo)
• palpitations (feeling your heartbeat)
• rapid heartbeat
• cough
• throat irritation
• speech difficulties
• difficulty in passing urine (water)
• skin reactions
Rare, may affect up to 1 in 1000 people
• Serious allergic reaction which causes difficulty in breathing or dizziness
• Allergic reactions such as hives and itching^ swelling of the face, lips and tongue
• decreased potassium levels
• mental disorders
• sweating
• eye pain or other problems with the eyes including blurring of vision, mydriasis (the excessive dilation of the pupil of the eye) and glaucoma (increased pressure in the eyes)
• irregular heart beat
• reduced blood pressure
• heart failure
• breathing difficulties and shortness of breath
• swelling of the throat
• diarrhoea, constipation, being sick (vomiting) or other problems with your digestive system
• taste change
• dental caries
• muscular pains
• weakness and cramps
• dry throat
• mouth oedema
• stomatitis
Very Rare, may affect up to 1 in 10000 people
• increased blood pressure
Although it is not known exactly how often this happens, some people might experience chest pain (due to problems such as angina). Tell your doctor as soon as possible if you develop these symptoms whilst receiving treatment with Zerseos, but do not stop using this medicine unless your doctor told you to do so.
You may also get unusually low levels of potassium in your blood (called ‘hypokalemia’).
If this happens, your doctor will keep checking your potassium levels.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard. By reporting side affects you can help provide more information on the safety of this medicine.
Page 3
Keep Zerseos out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, pouch and ampoule label after Exp. The expiry date refers to the last day of that month.
Do not refrigerate or freeze. Do not store above 25°C.
For single use only. Use immediately after first opening the ampoule. Discard immediately after first use. Partly used, opened or damaged ampoules should be disposed in accordance with local requirements. Keep ampoules in the outer pouch or carton in order to protect from light. Do not use this medicine if you notice that the nebuliser solution is cloudy.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Zerseos contains
• The active substances are ipratropium bromide and salbutamol. Each ampoule (2.5 ml dose) contains 0.5 mg of ipratropium bromide (as 525 micrograms ipratropium bromide monohydrate) and 2.5 mg of salbutamol (as sulphate).
• The other ingredients are sodium chloride, water for injections and sulfuric acid (for pH adjustment). What Zerseos looks like and contents of the pack
The single dose container is a Plastic Form fill Seal (FFS) ampoule containing 2.5 ml of colourless nebuliser solution. Five plastic ampoules are over wrapped in a triple laminated pouch and then packed into cardboard cartons containing 10, 20, 40, 60, 80 or 100 ampoules.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Cipla (EU) Limited, Hillbrow House, Hillbrow Road, Esher, Surrey, KT10 9NW, United Kingdom Manufacturer
S&D Pharma CZ, spol. s r.o, Plsnicka 22, 142 00 Praha 4, Czech Republic
Cipla (EU) Ltd, 4th Floor, 1 Kingdom Street, London, W2 6BY, United Kingdom
This medicinal product is authorised in the Member States of the EEA under the following names
|
The Netherlands |
Ipratropiumbromide/Salbutamol Cipla 0,5/2,5 mg per 2,5 ml, verneveloplossing |
|
United kingdom |
Zerseos 0.5 mg/2.5 mg per 2.5 ml Nebuliser solution |
|
Italy |
Ipratropio bromuro e Salbutamolo Cipla |
|
Greece |
Ipratropiumbromide/Salbutamol Cipla 0,5mg /2,5 mg per 2,5 ml Aia^up,a yia siorcvo'q p,s SKVsurorq |
|
Sweden |
Ipratropiumbromid/Salbutamol Cipla |
|
Denmark |
Ipratropiumbromid/Salbutamol Cipla |
|
Finland |
Ipratropiumbromidi/Salbutamoli Cipla 0,5 mg/2,5 mg sumutinliuos |
|
Hungary |
Ipratropium-bromid / Szalbutamol Cipla 0,5 mg/2,5 mg per 2,5 ml Oldat porlasztasra |
|
Romania |
Ipratropiu / Salbutamol Cipla 0,5 mg / 2,5 mg solutie de inhalat prin nebulizator |
|
Belgium |
Bromure d’ipratropium/Salbutamol Cipla 0,5 mg/2,5 ml + 2,5 mg/2,5 ml, solution pour inhalation par nebuliseur |
|
Luxembourg |
Bromure d'ipratropium / Salbutamol Cipla 0,5 mg/2,5 mg per 2,5 ml Solution pour inhalation par nebuliseur |
|
Austria |
Ipratropiumbromid/Salbutamol Cipla 0,5 mg/2,5 mg - Losung fur einen Vernebler |
|
Croatia |
Ipratropijev bromid / Salbutamol Cipla 0,5 mg + 2,5 mg / 2,5 ml otopina za atomizator |
|
Czech Republic |
Ipratropium Bromide/Salbutamol Cipla 0,5 mg/2,5 ml + 2,5 mg/2,5 ml Roztok k rozprasovani |
|
Slovak republic |
Ipratropii bromidum/ Salbutamol Cipla 0,5 mg/2,5 mg per 2,5 ml Roztok pre rozprasovac |
|
Slovenia |
Ipratropijev bromid/salbutamol Cipla 0,5 mg/2,5 mg v 2,5 ml inhalacijska raztopina za nebulator |
|
Bulgaria |
unpaTponueB 6poMHg / can6yTaMon Cnnaa 0,5 mg/2,5 mg per 2,5 ml Pa3TBop 3a He6y^H3aTop |
This leaflet was last approved in 09/2014.
Cipla
Page 4