Zibor 2 500 Iu Anti-Xa/0.2 Ml Solution For Injection
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 1 of 7 |
Package leaflet: Information for the user Zibor 2,500 IU anti-Xa/0.2 ml solution for injection in pre-filled syringes
Bemiparin sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4..
What is in this leaflet
1. What Zibor is and what is it used for
2. What you need to know before you use Zibor
3. How to use Zibor
4. Possible side effects
5. How to store Zibor
6. Contents of the pack and other information
1. What Zibor is and what it is used for
The active ingredient in Zibor is bemiparin sodium, which belongs to a group of medicines called anticoagulants. These help to stop blood from clotting in the blood vessels.
Zibor is used to prevent dangerous blood clots, which have formed in, for example, the veins of the legs and/or the lungs, which can occur if you are undergoing general surgery, and it is also used to prevent blood clots forming in the tubing of the dialysis machine.
2. What you need to know before you use Zibor Do not use Zibor:
- if you are allergic to bemiparin sodium or any other ingredients of this medicine (listed in section 6).
- if youhave had an allergic reaction after being given any medicine containing heparin.
- if you are allergic to any substance derived from pigs.
- if you suffer from Heparin Induced Thrombocytopenia (HIT), a condition that produces a severe decrease in your number of blood-clotting cells (platelets), (or, as a result of HIT, you suffer from another condition called, Disseminated Intravascular Coagulation (DIC), where your blood-clotting cells would clump together if Zibor is used.
- if you suffer from a condition known as endocarditis (inflammation of the lining of the heart and heart valves).
- if you suffer from any condition which results in a tendency to bleed excessively.
- if you suffer from a serious liver and/or pancreas disease.
- if you have any damage to your internal organs which may lead to a high risk of internal bleeding (for example, an active stomach ulcer, cerebral aneurisms [swelling in the artery walls in the brain], or brain tumours).
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 2 of 7 |
- if you have suffered from a brain haemorrhage.
- if you have had, have or are going to have injuries to or operations on, your brain, spine, eyes and/or ears within the last 2 months.
- if you are being treated with Zibor, you must not have epidural or spinal anaesthesia (an anaesthetic injected into your spine) because it could be dangerous. Therefore, make sure your doctor knows that you are being treated with Zibor before any surgery.
Warnings and precautions
Talk to your doctor before using Zibor
- if you suffer from liver disease.
- if you suffer from kidney disease your physician may consider to conduct a special monitoring.
- if your blood pressure is high and/or difficult to control.
- if you have ever had a stomach ulcer which is no longer active.
- if you suffer from thrombocytopenia, a condition where there are fewer than normal blood-clotting cells (platelets) in your blood, making you bruise and bleed easily.
- if you have kidney stones and/or bladder stones.
- if you suffer from any condition which may cause you to bleed more easily.
- if you suffer from eye problems, due to problems in your blood vessels.
- if you suffer from diabetes.
- if your blood tests have shown that you have high levels of potassium in your blood.
- make doubly sure your doctor knows you are being treated with Zibor if you are going to have a lumbar puncture (a puncture in the lower part of the spine for laboratory tests).
Other medicines and Zibor
Check with your doctor if you think you may already be taking:
- any medicine, which is injected into a muscle, because such injections must be avoided during treatment with Zibor.
- other anticoagulants such as warfarin and/or acenocoumarol (Vitamin K antagonists), to treat and/or prevent blood clots.
- non-steroidal anti-inflammatory drugs, such as ibuprofen, for example, for arthritis.
- steroids, such as prednisolone, to treat inflammatory diseases, such as arthritis.
- platelet inhibitors, such as aspirin, ticlopidine or clopidogrel, to prevent blood clots.
- medicines which can increase levels of potassium in your blood, such as some diuretics (water pills) and anti-hypertensives (used to reduce blood pressure).
- medicines to increase your blood volume, such as dextran.
- an injected drug used to treat heart problems, called glyceryl nitrate.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other
medicines, including medicines obtained without a prescription.
Special tests you may need
- Some patients may need to have the level of blood clotting cells in their blood checked. Your doctor will decide whether this is necessary and when (e.g. Before treatment, on the first day of treatment, then every 3-4 days and at the end of treatment).
- If you suffer from certain conditions (diabetes, kidney disease) or if you are taking medicines to prevent the loss of potassium, your doctor may check the potassium level in your blood.
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 3 of 7 |
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Zibor has no effect on the ability to drive or operate machinery.
3. How to use Zibor
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
To prevent blood clots in the veins when undergoing general surgery:
- Zibor 2,500 IU is usually administered by a doctor or a nurse by subcutaneous injection (it means is injected under the skin, usually into a skin fold in your abdomen or the upper part of your thigh). You will be given one dose of the product (the contents of the syringe) before or after your operation. The following days you will be given one dose (the contents of one syringe) every day. Your doctor will tell you how long you should be given this medicine for.
Prevention of clotting during haemodialysis:
- When used in haemodialysis, Zibor 2,500 IU is usually administered by injecting one bolus dose (the contents of the syringe) into the arterial side of the dialysis machine.
Zibor is usually injected under your skin, usually into a skin fold at the side of your waist (abdomen) or in the upper part of the thigh. Your doctor or nurse will usually administer the injection in hospital. You may need to continue to receive Zibor when you return home.
- This medicine must never be injected into a muscle or mixed with any other injection.
- It is usually given once a day.
- Your doctor will tell you for how long you should be given this medicine (usually for approximately 7 - 10 days).
- If your doctor has told you that you can inject this medicine yourself, please follow your doctor’s instructions extremely carefully. (See below “How do I inject Zibor?”).
Elderly patients (65 years old and above) are normally given the same dosages as other adult patients. If you have liver or kidney problems, please tell your doctor who may wish to keep a close eye on you.
Use in children (under 18 years of age)
Zibor is not recommended for children.
How do I inject Zibor?
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 4 of 7 |
Zibor should never be injected into muscle because that could cause bleeding into the muscle. You should receive instructions on the correct way to use this medicine and the correct technique for self-injection before you give yourself an injection for the first time.
These instructions should be given by a doctor or other qualified healthcare professional.
You should follow these steps:
- Wash your hands well and sit or lie in a comfortable position.
- Choose an area of the waist, at least 5 centimetres away from your belly button and from existing scars or bruises and clean the skin carefully.
- Use different places for the injection on different days, for example, first on the left hand side, next time on the right.
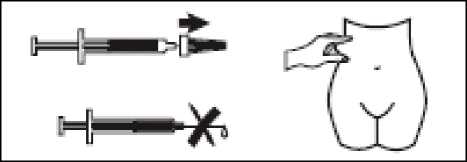
- Pull the needle cap off the Zibor syringe.
- To keep the needle sterile, make sure it doesn’t touch anything.
- This pre-filled syringe is now ready for use.
- Before injecting, do not push the plunger to get rid of any air bubbles, because you might lose the medicine
- Hold the syringe in one hand and with your other hand, using your forefinger and thumb, gently pinch the area of skin which you’ve cleaned and make a skin fold.
- Insert the full length of the needle into the folded skin, straight in at a 90° angle.
- Press down on the plunger, making sure you hold the skin fold in position throughout the injection.
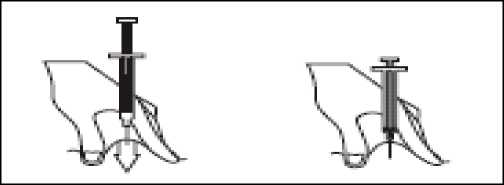
- Remove the needle by pulling it straight out and let go of the skin fold.
- Don’t rub the skin where you put the needle in. This will help to avoid bruises.
- Don’t try to put the needle cap back on the syringe. Just drop it (needle first) into a sharps bin, close the container lid tightly and place it out of reach of children.
- If you get the impression that the dose is either too strong (for example, you are experiencing unexpected bleeding) or too weak (for example, the dose doesn’t seem to be working), talk to your doctor or pharmacist.
In some package sizes, the prefilled syringe may be combined to a safety device system which is to be activated after injection to reduce the risk of needlestick injuries.
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 5 of 7 |
For syringes with safety device system: Orient the needle away from you and anyone else who is present, activate the safety system by pressing firmly on the plunger rod. The protective sleeve will automatically cover the needle and will produce an audible click which confirms the activation of the device.
Immediately discard the syringe by throwing it into the nearest sharps bin (the needle in), close the container lid tightly and place the container out of the reach of children.
If you use more Zibor than you should
This may result in bleeding. If this happens, tell your doctor immediately or go immediately to the casualty department at your nearest hospital with this leaflet.
If you forget to use Zibor
Do not take a double dose to make up for a forgotten dose. You should consult your doctor as soon as possible so that he may tell you what to do.
If you stop using Zibor
Always check with your doctor before you stop using this medicine.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Zibor and tell the doctor or nurse immediately (or go immediately to your nearest Casualty or Accident and Emergency Department), if you notice any of the following side effects:
Common (these may affect up to 1 in 10 patients):
- Unusual or unexpected bleeding, for example blood in your urine and/or stools.
Rare (these may affect up to 1 in 1,000 patients):
- Severe decrease in your number of blood-clotting cells (thrombocytopenia type II) which can lead to bruising, bleeding in the mouth, gums and nose, rash.
- Dark painful skin reactions at the injection site (Cutaneous necrosis).
- Intra-spinal haematomas following spinal or lumbar anaesthesia (back pain, numbness and weakness in the lower limbs, bowel or bladder dysfunction). These haematomas could cause various degrees of neurological impairment, including prolonged or permanent paralysis.
- Serious allergic reactions (raised temperature, shivering, breathlessness, swelling in your vocal cords, light-headedness, sweating, nettle rash/hives, itchy skin, low blood pressure, hot flushes, flushing, black out, contraction of the bronchial tube, swelling of the larynx.
Other side effects:
Very Common (affect more than 1 in 10 patients):
- Bruising, blotchy skin, itching and some pain at the places where the medicine was injected.
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 6 of 7 |
Common (these may affect up to 1 in 10 patients):
- A slight and temporary increase in certain enzymes (transaminases), which would show up in blood tests.
Uncommon (these may affect up to 1 in 100 patients):
- Mild and transient temporary decrease in your number of blood-clotting cells (thrombocytopenia type I), which would show up in blood tests.
- Mild allergic skin reactions: skin rash, nettle rash/hives, weals.
Not known (cannot be estimated from the available data):
- Increase in potassium levels, which could show up in blood tests.
Brittle bones (osteoporosis) may develop through use of this or similar medicines over a long period of time. The frequency is unknown.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme, Website: www.mhra.gov .uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Zibor
Keep this medicine out of the sight and reach of children.
Do not store above 30 °C. Do not freeze.
Do not use this medicine if you notice:
- the protective package has already been opened.
- the protective package is damaged.
- the medicine in the syringe appears cloudy.
- it contains small particles.
After the blister containing the syringe has been opened, the medicine should be used immediately.
Expiry date
Do not use this medicine after the expiry date which is stated on the carton.
The expiry date refers to the last day of that month.
Disposal
This medicine comes in single-dose syringes.
Drop used syringes into a sharps bin.
Do not keep them after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Zibor contains
- The active substance is bemiparin sodium.
|
Zibor 2,500 IU/0.2 ml - Patient Information Leaflet |
March 2015 Page 7 of 7 |
- The other ingredient is water for injections.
What Zibor looks like and contents of the pack
The medicine contained in the syringes is a clear, colourless or slightly yellowish solution, free of particles.
Zibor 2,500 IU is available in packs of 2, 6, 10, 30 and 100 pre-filled syringes containing 0.2 ml of solution for injection.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
FROSST IBERICA, S.A.
Via Complutense, 140
28805 Alcala de Henares (Madrid)
Manufacturer
ROVI CONTRACT MANUFACTURING, S.L. Julian Camarillo, 35 28037 MADRID - SPAIN
Laboratorios Farmaceuticos ROVI, S.A.
Julian Camarillo, 35 28037 MADRID - SPAIN
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria
Czech Republic
Estonia
Greece
Hungary
Ireland
Italy
Latvia
Lithuania
Poland
Portugal
Slovakia
Slovenia
Spain
United Kingdom
Ivor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Ivor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Ivor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Ivor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml Phivor 2,500 IU Anti Xa/0.2 ml Zibor 2,500 IU Anti Xa/0.2 ml
This leaflet was last revised in
Zibor2500 PIL