Zibor 25 000 Iu Anti-Xa/Ml Sln Fr Injctn In Pre-Fill Syringe
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Zibor 25,000 IU anti-Xa/ml solution for injection
Bemiparin sodium
Read all of this leaflet carefully before you start using this medicine
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Zibor is and what is it used for
2. Before you use Zibor
3. How to use Zibor
4. Possible side effects
5. How to store Zibor
6. Further information
1. WHAT ZIBOR IS AND WHAT IT IS USED FOR
The active ingredient in Zibor is bemiparin sodium, which belongs to a group of medicines called anticoagulants. These help to stop blood from clotting in the blood vessels.
Zibor is used to treat dangerous blood clots, which have formed in, for example, the veins of the legs and/or the lungs (deep vein thrombosis and/or pulmonary embolism).
2. BEFORE YOU USE ZIBOR Do not use Zibor:
- if you have had an allergic reaction after being given any medicine containing bemiparin sodium or heparin.
- if you are allergic to any substance derived from pigs.
- if you suffer from Heparin Induced Thrombocytopenia (HIT), a condition that produces a severe decrease in your number of blood-clotting cells (platelets), (or, as a result of HIT, you suffer from another condition called, Disseminated Intravascular Coagulation (DIC), where your blood-clotting cells would clump together if Zibor is used.
- if you suffer from a condition known as endocarditis (inflammation of the lining of the heart and heart valves).
- if you suffer from any condition which results in a tendency to bleed excessively.
- if you suffer from a serious liver and/or pancreas disease.
- if you have any damage to your internal organs which may lead to a high risk of internal bleeding (for example, an active stomach ulcer, cerebral aneurisms [swelling in the artery walls in the brain], or brain tumours).
- if you have suffered from a brain haemorrhage.
- if you have an injury to, or are going to have an operation on, your brain, spine, eyes and/or ears.
- if you are being treated with Zibor, you must not have epidural or spinal anaesthesia (an anaesthetic injected into your spine) because it could be dangerous. Therefore, make sure your doctor knows that you are being treated with Zibor before any surgery.
Take special care with Zibor:
- if you suffer from liver or kidney disease.
- if your blood pressure is high and/or difficult to control.
- if you have ever had a stomach ulcer which is no longer active.
- if you suffer from thrombocytopenia, a condition where there are fewer than normal blood-clotting cells (platelets) in your blood, making you bruise and bleed easily.
- if you have kidney stones and/or bladder stones.
- if you suffer from any condition which may cause you to bleed more easily.
- if you suffer from eye problems, due to problems in your blood vessels.
- if you suffer from diabetes.
- if your blood tests have shown that you have high levels of potassium in your blood.
- make doubly sure your doctor knows you are being treated with Zibor if you are going to have a lumbar puncture (a puncture in the lower part of the spine for laboratory tests).
Ask your doctor if you are unsure whether or not you have any of these conditions.
Using other medicines
Check with your doctor if you think you may already be taking:
- any medicine, which is injected into a muscle, because such injections must be avoided during treatment with Zibor.
- other anticoagulants such as warfarin and/or acenocoumarol (Vitamin K antagonists), to treat and/or prevent blood clots.
- non-steroidal anti-inflammatory drugs, such as ibuprofen, for example, for arthritis.
- steroids, such as prednisolone, to treat inflammatory diseases, such as arthritis.
- platelet inhibitors, such as aspirin, ticlopidine or clopidogrel, to prevent blood clots.
- medicines which can increase levels of potassium in your blood, such as some diuretics (water pills) and anti-hypertensives (used to reduce blood pressure).
- medicines to increase your blood volume, such as dextran.
- an injected drug used to treat heart problems, called glyceryl nitrate.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Special tests you may need
- Some patients may need to have the level of blood clotting cells in their blood checked. Your doctor will decide whether this is necessary and when (e.g. Before treatment, on the first day of treatment, then every 3-4 days and at the end of treatment).
- If you suffer from certain conditions (diabetes, kidney disease) or if you are taking medicines to prevent the loss of potassium, your doctor may check the potassium level in your blood.
Pregnancy and breast-feeding
Before starting treatment with this medicine, tell your doctor:
- If you think you are or could be pregnant.
- If you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Zibor has no effect on the ability to drive or operate machinery.
3. HOW TO USE ZIBOR
Always use Zibor exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Zibor is usually injected under your skin, usually into a skin fold at the side of your waist (abdomen) or in the upper part of the thigh. Your doctor or nurse will usually administer the injection in hospital. You may need to continue to receive Zibor when you return home.
- This medicine must never be injected into a muscle or mixed with any other injection.
- It is usually given once a day.
- Your doctor will tell you for how long you should be given this medicine (usually for approximately 5-9 days).
- If your doctor has told you that you can inject this medicine yourself, please follow your doctor’s instructions extremely carefully. (See below “How do I inject Zibor?”).
Adults (aged 18 to 64)
Your daily injection dose will depend upon your body weight. If you weigh:
- less than 50 Kilos, the dose will be 0.2 millilitres (= 5,000 IU).
- between 50 and 70 kilos, the dose will be 0.3 millilitres (=7,500 IU).
- between 71 and 100 kilos, the dose will be 0.4 millilitres (=10,000 IU).
- more than 100 kilos, the dose will be adjusted, depending on your exact weight, to the equivalent of 115 IU a day for each kilogram you weigh.
IU: The potency of this medicine is described in International anti-Xa activity units.
Elderly patients (65 years old and above) are normally given the same dosages as other adult patients. If you have liver or kidney problems, please tell your doctor who may wish to keep a close eye on you.
Children (under 18 years of age): Zibor is not recommended for children.
How do I inject Zibor?
Zibor should never be injected into muscle because that could cause bleeding into the muscle. You should receive instructions on the correct way to use this medicine and the correct technique for self-injection before you give yourself an injection for the first time.
These instructions should be given by a doctor or other qualified healthcare professional.
You should follow these steps:
- Wash your hands well and sit or lie in a comfortable position.
- Choose an area of the waist, at least 5 centimetres away from your belly button and from existing scars or bruises and clean the skin carefully.
- Use different places for the injection on different days, for example, first on the left hand side, next time on the right.
- Pull the needle cap off the Zibor syringe.
- To keep the needle sterile, make sure it doesn’t touch anything.
- This pre-filled syringe is now ready for use.
- Before injecting, do not push the plunger to get rid of any air bubbles, because you might lose the medicine
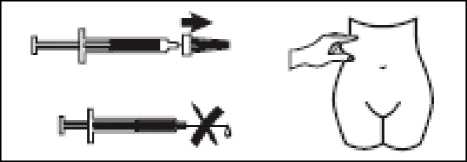
- Hold the syringe in one hand and with your other hand, using your forefinger and thumb, gently pinch the area of skin which you’ve cleaned and make a skin fold.
- Insert the full length of the needle into the folded skin, straight in at a 90° angle.
- Press down on the plunger, making sure you hold the skin fold in position throughout the injection.
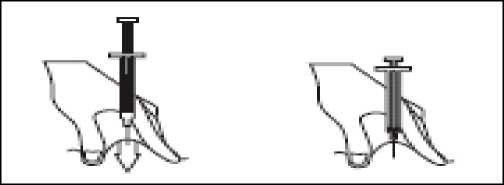
- Remove the needle by pulling it straight out and let go of the skin fold.
- Don’t rub the skin where you put the needle in. This will help to avoid bruises.
- Don’t try to put the needle cap back on the syringe. Just drop it (needle first) into a sharps bin, close the container lid tightly and place it out of reach of children.
- If you get the impression that the dose is either too strong (for example, you are experiencing unexpected bleeding) or too weak (for example, the dose doesn’t seem to be working), talk to your doctor or pharmacist.
If you use more Zibor than you should
This may result in bleeding. If this happens, tell your doctor immediately or go immediately to the casualty department at your nearest hospital with this leaflet.
If you forget to use Zibor
Do not take a double dose to make up for a forgotten individual dose. You should consult your doctor as soon as possible so that he may tell you what to do.
If you stop using Zibor
Always check with your doctor before you stop using this medicine.
If you have any further questions on the use of this product, ask your doctor.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Zibor can cause side effects, although not everybody gets them.
Stop using Zibor and tell the doctor or nurse immediately (or go immediately to your nearest Casualty or Accident and Emergency Department), if you notice any of the following side effects:
Common (these may affect up to 1 in 10 patients):
- Unusual or unexpected bleeding, for example blood in your urine and/or stools.
Rare (these may affect up to 1 in 1,000 patients):
- Severe decrease in your number of blood-clotting cells (thrombocytopenia type II) which can lead to bruising, bleeding in the mouth, gums and nose, rash.
- Dark painful skin reactions at the injection site (Cutaneous necrosis).
- Intra-spinal haematomas following spinal or lumbar anaesthesia (back pain, numbness and weakness in the lower limbs, bowel or bladder dysfunction). These haematomas could cause various degrees of neurological impairment, including prolonged or permanent paralysis.
- Serious allergic reactions (raised temperature, shivering, breathlessness, swelling in your vocal cords, light-headedness, sweating, nettle rash/hives, itchy skin, low blood
pressure, hot flushes, flushing, black out, contraction of the bronchial tube, swelling of the larynx.
Other side effects:
Very Common (affect more than 1 in 10 patients):
- Bruising, blotchy skin, itching and some pain at the places where the medicine was injected.
Common (these may affect up to 1 in 10 patients):
- A slight and temporary increase in certain enzymes (transaminases), which would show up in blood tests.
Uncommon (these may affect up to 1 in 100 patients):
- Mild and transient temporary decrease in your number of blood-clotting cells (thrombocytopenia type I), which would show up in blood tests.
- Mild allergic skin reactions: skin rash, nettle rash/hives, weals.
Not known (cannot be estimated from the available data):
- Increase in potassium levels, which could show up in blood tests.
Brittle bones (osteoporosis) may develop through use of this or similar medicines over a long
period of time. The frequency is unknown.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
5. HOW TO STORE ZIBOR
Keep out of the reach and sight of children.
Do not store above 25 °C. Do not freeze.
Do not use Zibor if you notice (and tell your doctor and pharmacists):
- the protective package has already been opened.
- the protective package is damaged.
- the medicine in the syringe appears cloudy.
- it contains small particles.
After the blister containing the syringe has been opened, the medicine should be used immediately.
Expiry date
Do not use Zibor after the expiry date, which is stated on the carton.
The expiry date refers to the last day of that month.
Disposal
This medicine comes in single-dose syringes.
Drop used syringes into a sharps bin.
Do not keep them after use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Zibor contains
- The active substance is bemiparin sodium.
- The other ingredient is water for injections.
What Zibor looks like and contents of the pack
The medicine contained in the syringes is a clear, colourless or slightly yellowish solution, free of particles.
Zibor 25,000 IU is available in packs of 2, 10, 30 and 100 pre-filled syringes containing 0.2 ml, 0.3 or 0.4ml of solution for injection.
Not all pack sizes may be marketed.
Each 0.2 ml syringe provides a dose of bemiparin sodium of 5,000 IU.
Each 0.3 ml syringe provides a dose of bemiparin sodium of 7,500 IU.
Each 0.4 ml syringe provides a dose of bemiparin sodium of 10,000 IU.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
FROSSTIBERICA, S.A.
Via Complutense 140, Alcala de Henares, 28805 Madrid, Spain
Manufactured by:
ROVI CONTRACT MANUFACTURING, S.L. Julian Camarillo, 35 28037 MADRID - SPAIN
This medicinal product is authorised in the Member States of the EEA under the following names:
Czech Republic
Estonia
Ireland
Italy
Latvia
Lithuania
Poland
Portugal
Slovakia
Slovenia
Spain
United Kingdom
Zibor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Ivor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Ivor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml Phivor 25,000 IU Anti Xa/ml Zibor 25,000 IU Anti Xa/ml