Zispin Soltab 15Mg Orodispersible Tablet
Package leaflet: Information for the patient
Read all of this leaflet carefully before
you start taking this medicine because
it contains important information for
you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• Ihis medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor or pharmacist. Ihis includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Zispin SolTab is and what it is used for
2. What you need to know before you take Zispin SolTab
3. How to take Zispin SolTab
4. Possible side effects
5. How to store Zispin SolTab
6. Contents of the pack and other information
Zispin SolTab is called antidepre Zispin SolTab is in adults.
Zispin SolTab wil it starts working, start feeling bette doctor if you do worse after 2 to in section 3 hea to start feeling
ne of a group of medicines ssants.
used to treat depressive illness
take 1 to 2 weeks before After 2 to 4 weeks you may r. You must talk to your not feel better or if you feel 4 weeks. More information is ding “When can you expect tter”.


be
2. What you need to know before you take Zispin SolTab
Do not take Zispin SolTab:
• if you are allergic to mirtazapine or any of the other ingredients of this medicine (listed in section 6). If so, you must talk to your doctor as soon as you can before taking Zispin Sollab.
• if you are taking or have recently taken (within the last two weeks) medicines called monoamine oxidase inhibitors (MAO-Is).
Warnings and precautions
lalk to your doctor or pharmacist before taking Zispin Sollab.
Children and adolescents
Zispin Sollab should normally not be used for children and adolescents under 18 years because efficacy was not demonstrated. Also, you should know that patients under 18 have an increased risk of side-effects such as suicide attempt, suicidal thoughts and hostility (predominantly aggression, oppositional behaviour and anger) when they take this class of medicines. Despire this, your docror may prescribe Zispin Sollab for patients under 18 because he/she decides that this is in their best interests. If your doctor has prescribed Zispin Sollab for a patient under 18 and you want to discuss this, please go back to your doctor. You should inform your doctor if any of the symptoms listed above develop or worsen when patients under 18 are taking Zispin Sollab. Also, the long-term safety effects concerning growth, maturation and cognitive and behavioural development of Zispin Sollab in this age group have not yet been demonstrated. In addition, significant weight gain has been observed in this age category more often when treated with Zispin Sollab compared with adults. Thoughts of suicide and worsening of your depression
If you are depressed you can sometimes have thoughts of harming or killing yourself. lhese may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
• if you have previously had thoughts about killing or harming yourself.
• if you are a young adult. Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
^ If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straightaway.
You may find it helpful to tell a relative or close friend that you are depressed, and ask them to read this leaflet. You might ask them to tell you if they think your depression is getting worse, or if they are worried about changes in your behaviour.
Also take special care with Zispin Sollab
• if you have, or have ever had one of the following conditions.
^ lell your doctor about these conditions before taking Zispin Sollab, if not done previously
- seizures (epilepsy). If you develop seizures or your seizures become more frequent, stop taking
Zispin Sollab and contact your doctor immediately:
- liver disease, including jaundice.
If jaundice occurs, stop taking
Zispin Sollab and contact your doctor immediately:
- kidney disease:
- heart disease, or low blood pressure:
- schizophrenia. If psychotic symptoms, such as paranoid thoughts become more frequent or severe, contact your doctor straightaway:
- manic depression (alternating periods of feeling elated/overactivity and depressed mood). If you start feeling elated or over-excited, stop taking Zispin Sollab and contact your doctor immediately:
- diabetes (you may need to adjust your dose of insulin or other antidiabetic medicines):
- eye disease, such as increased pressure in the eye (glaucoma):
- difficulty in passing water (urinating), which might be caused by an enlarged prostate:
- certain kinds of heart conditions
that may change your heart rhythm, a recent heart attack, heart failure, or take certain medicines that may affect the heart's rhythm.
• if you develop signs of infection such as inexplicable high fever, sore throat and mouth ulcers.
^ Stop taking Zispin Sollab and consult your doctor immediately for a blood test. In rare cases these symptoms can be signs of disturbances in blood cell production in the bone marrow. While rare, these symptoms most commonly appear after 4-6 weeks of treatment.
• if you are an elderly person. You could be more sensitive to the side-effects of antidepressants.
Other medicines and Zispin SolTab
lell your doctor or pharmacist f you are taking, have recently taken or might take any other medicines.
Do not take Zispin SolTab in c
• monoamine oxidase inhi
inhibitors). Also, do not take during the two weeks after stopped taking MAO inhibit taking Zispin Sollab, do not inhibitors during the next Examples of MAO inhibitors moclobemide, tranylcypro antidepressants) and selegi Parkinson’s disease).
Take care when taking Zispin Sollab in
combination with:
• antidepressants such as venlafaxine and L-tryptoph
(used to treat migraine), trai killer), linezolid (an antibioti (used to treat some psychiati methylene blue (used to
:ombinafion with: bitors (MAO Zispin Sollab you have ors. If you stop take MAO two weeks either. are
mine (both are line (used for
SS
RIs,
an or triptans madol (a pain, lithium
ric conditions), treat high
ic)
levels of methemoglobin in the blood) and St. John’s Wort - Hypericum perforatum preparations (a herbal remedy or depression). In very rare cases Zispin Sollab alone or the combination of Zispin Sollab with these medicines, can lead to a so-called serotonin syndrome. Some of the symptoms of this syndrome are: inexplicable fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering, overactive reflexes, restlessness, mood changes and unconsciousness. If you get a combination of these symptoms, talk to your doctor immediately.
• the antidepressant nefazodone. It can increase the amount of Zispin Sollab in your blood. Inform your doctor if you are using this medicine. It might be needed to lower the dose of Zispin Sollab, or when use of nefazodone is stopped, to increase the dose of Zispin Sollab again.
• medicines for anxiety or insomnia such as benzodiazepines:
medicines for schizophrenia such as olanzapine:
medicines for allergies such as cetirizine: medicines for severe pain such as morphine.
In combination with these medicines Zispin Sollab can increase the drowsiness caused by these medicines.
• medicines for infections: medicines for bacterial infections (such as erythromycin, medicines for fungal infections
(such as ketoconazole) and medicines for HIV/AIDS (such as HIV-protease inhibitors) and drugs for stomach ulcers (such as cimetidine).
In combnation with Zispin Sollab these medicines can increase the amount of Zispin Sollab in your blood. Inform your doctor if you are using these medicines.
It might be needed to lower the dose of Zispin Sollab, or when these medicines are stopped, to increase the dose of Zispin Sollab again.
• medicines for epilepsy such as carbamazepine and phenytoin: medicines for tuberculosis such as rifampicin.
In combnation with Zispin Sollab these medicines can reduce the amount of Zispin Sollab in your blood. Inform your doctor if you are using these medicines.
It might be needed to increase the dose of Zispin Sollab, or when these medicines are stopped to lower the dose of Zispin Sollab again.
• medicines to prevent blood clotting
such as warfarin.
Zispin Sollab can increase the effects of warfarin on the blood. Inform your doctor if you are using this medicine. In case of combination it is advised that a doctor monitors your blood carefully.
• medicines that may affect the heart’s rhythm such as certain antibiotics and some anti-psychotics.
Zispin SolTab with food and alcohol
You may get drowsy if you drink alcohol while you are taking Zispin Sollab.
You are advised not to drink any alcohol.
You can take Zispin Sollab with or without food.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Limited experience with Zispin Sollab administration to pregnant women does not
now turn over >•
indicate an increased should be exercised
risk. However, caution when used during
pregnancy.
If you use Zispin SolTt birth, your baby shoul possible adverse effe1 When taken during drugs (SSRIs) may in serious condition in pulmonary hypertens (PPHN), making the appear bluish. These begin during the first is born. If this happen should contact your immediately.
ab until, or shortly before d be supervised for cts.
pregnancy, similar crease the risk of a babies, called persistent ion of the newborn baby breathe faster and symptoms usually 24 hours after the baby s to your baby you midwife and/or doctor
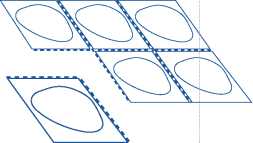
Tear off one tablet pocket
ch blister contains six tablet po> e separated by perforations. Tear blet pocket along the dotted
ckets, which off one s (Figure 1).
line:
The most likely Zispin SolTab (w alcohol) are dro' increased hear
possible overdo: your heart rhyth and/or fainting a life-threatenin de Pointes.
signs of an overdose of ithout other medicines or wsiness, disorientation and ■t rate. The symptoms of a se may include changes to m (fast, irregular heartbeat) which could be symptoms of g condition known as Torsade
Driving and using
Zispin SolTab can aff or alertness. Make not affected before y machinery. If your do' Zispin SolTab for a p> make sure the cone is not affected before (e.g. on bicycle).
machines
ect your concentration re these abilities are ou drive or operate ctor has prescribed atient under 18 years entration and alertness participation in traffic
Fig. 1.
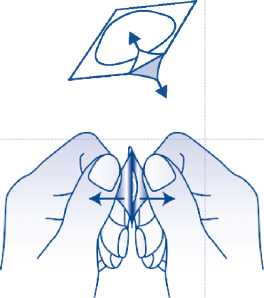
Peel off the lid
Carefully peel off the lidding foil, starting in the : rner indicated by the arrow (Figures 2 and 3).
If you forget
If you are supp a day
• Do not take a forgotten the normal
ZispinSolTaboro contain sugar sph sucrose.
Zispin SolTab orodisp sugar spheres, contai been told by your do intolerance for some doctor before taking
dispersible tablets eres, containing
ersible tablets contain ining sucrose. If you have ctor that you have an sugars, contact your this medicinal product.
Zispin Soltab orodispersible tablets contain aspartame, a source of phenylalanine.
Zispin SolTab orodisp aspartame, a source be harmful for peop
Fig. 2.
ersible tablets contain of phenylalanine. May e with phenylketonuria.
3. How to take Zispin SolTab
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
How much to take The recommended starting dose is 15 or 30 mg every day. Your doctor may advise you to increase your dose after a few days to the amount that is best for you (between 15 and 45 mg per day). The dose is usually the same for all ages. However, if you are an elderly person or if you have renal or liver disease, your doctor may adapt the dose.
When to take Zispin SolTab
•+ Take Zispin SolTab at the same time each day.
It is best to take Zispin SolTab as a single dose before you go to bed. However your doctor may suggest to split your dose of Zispin SolTab - once in the morning and once at night-time before you go to bed. The higher dose should be taken before you go to bed.
Take the orodispersible tablet as follows
Take your tablets orally.
1. Do not crush the orodispersible tablet
In order to prevent crushing the orodispersible tablet, do not push against the tablet pocket (Figure A).
Fig. 3.
4. Take out the orodispersible tablet
Take out the orodispersible tablet with dry hands and place it on the tongue.
(Figure 4).
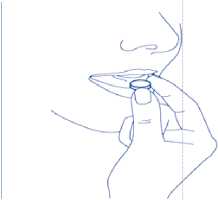
Fig. 4.
It will rapidly disintegrate and can be swallowed without water.
to take Zispin SolTab
osed to take your dose once
a double dose to make up for dose. Take your next dose at ime.
If you are supposed to take your dose twice a day
if you have forgotten to take your morning dose, simply take it together with your evening dose.
if you have forgotten to take your evening dose, do not take it with the next morning dose; just skip it and continue with your normal morning and evening doses.
• if you have forgotten to take both doses, do not attempt to make up for the missed doses. Skip both doses and continue the next day with your normal morning and evening doses.
If you stop taking Zispin SolTab
•+ Only stop taking Zispin SolTab in consultation with your doctor.
If you stop too early, your depression might come back. Once you are feeling better, talk to your doctor. Your doctor will decide when treatment can be stopped.
Do not suddenly stop taking Zispin SolTab, even when your depression has lifted. If you suddenly stop taking Zispin SolTab you may feel sick, dizzy, agitated or anxious, and have headaches. These symptoms can be avoided by stopping gradually. Your doctor will tell you how to decrease the dose gradually.
If you have any use of this medi pharmacist.
4. Possible side effects
further questions on the cine, ask your doctor or
Like all medicin side effects, alth them.
es, this medicine can cause ough not everybody gets
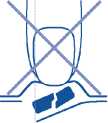
Fig. A.
When can you expect to start feeling better
Usually Zispin SolTab will start working after 1 to 2 weeks and after 2 to 4 weeks you may start to feel better.
It is important that, during the first few weeks of the treatment, you talk with your doctor about the effects of Zispin SolTab:
•+ 2 to 4 weeks after you have started taking Zispin SolTab, talk to your doctor about how this medicine has affected you.
If you still don’t feel better, your doctor may prescribe a higher dose. In that case, talk to your doctor again after another 2 to 4 weeks. Usually you will need to take Zispin SolTab until your symptoms of depression have disappeared for 4 to 6 months.
If you take more Zispin SolTab than you should
•+ If you or someone else has taken too much Zispin SolTab, call a doctor straightaway.
If you experience any of the following serious side effects, stop taking mirtazapine and tell your doctor immediately.
Uncommon (may affect up to 1 in 100 people):
• feeling elated or emotionally ‘high’ (mania)
Rare (may affect up to 1 in 1,000 people):
• yellow colouring of eyes or skin; this may suggest disturbance in liver function (jaundice)
Not known (frequency cannot be estimated from the available data):
• signs of infection such as sudden unexplainable high fever, sore throat and mouth ulcers (agranulocytosis).
In rare cases mirtazapine can cause disturbances in the production of blood cells (bone marrow depression). Some people become less resistant to infection because mirtazapine can cause a temporary shortage of white blood cells (granulocytopenia). In rare cases mirtazapine can also cause a shortage of red and white blood cells, as well as blood platelets (aplastic anemia), a shortage of blood platelets (thrombocytopenia) or an increase in the number of white blood cells (eosinophilia).
• epileptic attack (convulsions)
• a combination of symptoms such as inexplicable fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering, overactive reflexes, restlessness, mood changes, unconsciousness and increased salivation. In very rare cases these can be signs of serotonin syndrome.
• thoughts of harming or killing yourself
• severe skin reactions (Stevens-Johnson Syndrome, toxic epidermal necrolysis)
Other possible side effects with mirtazapine are:
Very common (may affect more than 1 in 10 people):
increase in appetite and weight gain drowsiness or sleepiness headache dry mouth
Common (may affect up to 1 in 10 people): lethargy dizziness
shakiness or tremor nausea diarrhoea vomiting constipation
rash or skin eruptions (exanthema) pain in your joints (arthralgia) or muscles (myalgia) back pain
feeling dizzy or faint when you stand up suddenly (orthostatic hypotension) swelling (typically in ankles or feet) caused by fluid retention (oedema) tiredness vivid dreams confusion feeling anxious sleeping problems Uncommon (may affect up to 1 in 100 people):
• abnormal sensation in the skin e.g. burning, stinging, tickling or tingling (paraesthesia)
• restless legs
• fainting (syncope)
• sensations of numbness in the mouth (oral hypoaesthesia)
• low blood pressure
• nightmares
• feeling agitated
• hallucinations
• urge to move
Rare (may affect up to 1 in ,000 people):
• muscle twitching or contractions (myoclonus)
• aggression
• abdominal pain and nausea; this may suggest inflammation of the pancreas (pancreatitis)
Not known (frequency cannot be estimated from the available data):
• abnormal sensations in the mouth (oral paraesthesia)
swelling in the mouth (mouth oedema) swelling throughout the body (generalized oedema) localized swelling hyponatraemia
inappropriate anti-diuretic hormone secretion
severe skin reactions (dermatitis bullous, erythema multiforme) sleep walking (somnambulism) speech disorder Additional side effects in children and adolescents
In children under 18 years the following adverse events were observed commonly in clinical trials: significant weight gain, hives and increased blood triglycerides
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible sid' effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Zispin SolTab
Keep this medicine out of the sight and reach of children.
ate er.
this medicine after the expiry di tated on the carton and the blist date refers to the last day of that
Do not use which is s The expiry month.
Store in the original package in order to protect from light and moisture.
Do not throw away any medicines via wastewater or household waste.
Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Zispin SolTab contains
• The active substance is mirtazapine. Zispin SolTab 15 mg orodispersible tablets contain 15 mg mirtazapine per orodispersible tablet.
• The other ingredients are sugar spheres, hypromellose, povidone K30, magnesium stearate, basic butylated methacrylate copolymer, aspartame (E951), anhydrous citric acid, crospovidone (type A), mannitol (E421), microcrystalline cellulose, natural and artificial orange flavour (No. SN027512) and sodium hydrogen carbonate.
What Zispin SolTab looks like and
contents of the pack
Zispin SolTab are orodispersible tablets.
Zispin SolTab 15 mg orodispersible tablets are round, wh te, standard bevelled-edge tablets coded with ‘TZ1’ on one side.
The orodispersible tablets are packed in a child-resistant perforated unit dose blister.
For Zispin the followi 30, 48, 90 (not all pa
Marketin
SolTab 15 mg orodispersible tablets ng pack sizes are available: 6, 18, 96 and 180 orodispersible tablets ck sizes may be marketed).
g Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Merck Sharp & Dohme Limited,
Hertford Road, Hoddesdon,
Hertfordshire, EN11 9BU,
United Kingdom
Manufacturer
N.V. Organon,
Kloosterstraat 6, P.O.Box 20,
5340 BH Oss,
The Netherlands
This medic Member Zispin SolT( associated
Belgium
Denmark
Germany
Hungary
Iceland
Ireland
Italy
Luxembou Norway Spain Sweden United
inal product is authorised in the States of the EEA under tradenam ab and under the following invented names:
Remergon SolTab Remeron Smelt Remergil SolTab Remeron Remeron Smelt Zispin SolTab Remeron
rg Remergon SolTab
Remeron-S Rexer Flas Remeron-S Zispin SolTab
Kingdom
This leaflet was last revised June 2014
^MSD