Zofran Melt 8Mg
Package Leaflet: Information for the User
Zofran® melt 8mg
(ondansetron)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions about your illness or your medicine, ask your doctor, nurse or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
• The name of this medicine is Zofran melt 8mg but will be referred to as Zofran melt throughout the remainder of this leaflet.
What is in this leaflet:
1. What Zofran melt is and what it is used for
2. What you need to know before you take Zofran melt
3. How to take Zofran melt
4. Possible side effects
5. How to store Zofran melt
6. Contents of the pack and other information
1. What Zofran melt is and what it is used for
Zofran melt contains a medicine called ondansetron. This belongs to a group of medicines called anti-emetics. Zofran melt is a special type of Zofran tablet that dissolves very quickly when put on top of the tongue.
Zofran melt is used for:
• preventing nausea and vomiting caused by chemotherapy (in adults and children) or radiotherapy for cancer (adults only)
• preventing nausea and vomiting after surgery (adults only).
Ask your doctor, nurse or pharmacist if you would like any further explanation about these uses.
2. What you need to know before you take Zofran melt Do not take Zofran melt if:
• you are taking apomorphine (used to treat Parkinson's disease)
• you are allergic (hypersensitive) to ondansetron or any of the other ingredients in Zofran melt (listed in Section 6).
If you are not sure, talk to your doctor, nurse or pharmacist before taking Zofran melt.
Warnings and precautions
Check with your doctor, nurse or pharmacist before taking Zofran melt if:
• you have ever had heart problems (e.g. congestive heart failure which causes shortness of breath and swollen ankles)
• you have an uneven heart beat (arrhythmias)
• you are allergic to medicines similar to ondansetron, such as granisetron or palonosetron
• you have liver problems
• you have a blockage in your gut
• you have problems with the levels of salts in your blood, such as potassium, sodium and magnesium.
If you are not sure if any of the above apply to you, talk to your doctor, nurse or pharmacist before taking Zofran melt.
Other medicines and Zofran
Please tell your doctor, nurse or pharmacist if you are taking or have recently taken or might take other medicines.
This includes medicines that you buy without a prescription and herbal medicines. This is because Zofran can affect the way some medicines work. Also some other medicines can affect the way Zofran works.
In particular, tell your doctor, nurse or pharmacist if you are taking any of the following medicines:
• carbamazepine or phenytoin used to treat epilepsy
• rifampicin used to treat infections such as tuberculosis (TB)
• antibiotics such as erythromycin or ketoconazole
• anti-arrhythmic medicines used to treat an uneven heart beat
• beta-blocker medicines used to treat certain heart or eye problems, anxiety or prevent migraines
• tramadol, a pain killer.
• medicines that affect the heart (such as haloperidol or methadone)
• cancer medicines (especially anthracyclines and trastuzumab).
• SSRIs (selective serotonin reuptake inhibitors) used to treat depression and/or anxiety including fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram
• SNRIs (serotonin noradrenaline reuptake inhibitors) used to treat depression and/or anxiety including venlafaxine, duloxetine.
If you are not sure if any of the above applies to you, talk to your doctor, nurse or pharmacist before having Zofran melt.
Pregnancy and breast-feeding
It is not known if Zofran is safe during pregnancy. If you are pregnant, think you are pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking Zofran melt.
Do not breast-feed if you are taking Zofran. This is because small amounts pass into the mother's milk. Ask your doctor or midwife for advice.
Important information about some of the ingredients of Zofran melt
• This medicine contains aspartame (which is a source of phenylalanine). If you have an inherited illness called phenylketonuria speak to your doctor before taking this medicine.
• This medicine contains sodium methyl para-hydroxybenzoate and sodium propyl para-hydroxybenzoate. This may cause allergic reactions (which could be delayed).
3. How to take Zofran melt
Always take Zofran melt exactly as your doctor has told you. You should check with your doctor, nurse or pharmacist if you are not sure. The dose you have been prescribed will depend on the treatment you are having.
To prevent nausea and vomiting from chemotherapy or radiotherapy
On the day of chemotherapy or radiotherapy
• the usual adult dose is 8mg taken one or two hours before treatment and another 8mg twelve hours after.
On the following days
• the usual adult dose is 8mg twice a day
• this may be given for up to 5 days.
Children aged over 6 months and adolescents:
The doctor will decide the dose depending on the child's size (body surface area) or weight. Look at the label for more information.
• the usual dose for a child is up to 4mg twice a day
• this can be given for up to 5 days.
To prevent nausea and vomiting after an operation
The usual adult dose is 16mg before your operation
Children aged over 1 month and adolescents:
It is recommended that Zofran is given as an injection.
Patients with moderate or severe liver problems
The total daily dose should not be more than 8mg.
POM
How to remove Zofran melt from the blister and take the
medicine
• Do not take a Zofran melt tablet out from its blister until you are ready to take it.
• Before you take the Zofran melt make sure the foil packaging has not been pierced.
• Important: Do not try to push Zofran melt through the foil top like a usual tablet. This is because Zofran melt is fragile and will break.
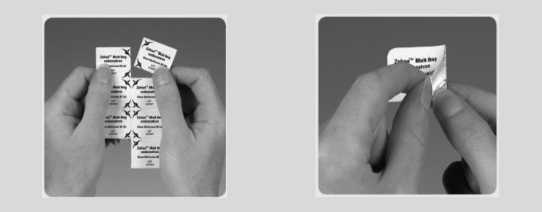
Tear off one Zofran Peel back the foil, as
melt in its blister 2 shown by the arrow

3 Gently push out the A Place the Zofran melt on
Zofran melt tablet. 4 top of the tongue.
It will dissolve very quickly. Then you can swallow as normal.
Zofran melt should start to work within one or two hours of taking a dose.
If you are sick (vomit) within one hour of taking a dose
• take the same dose again
• otherwise, do not take more Zofran melt than the label says.
If you continue to feel sick, tell your doctor or nurse.
If you take more Zofran melt than you should
If you or your child take more Zofran than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you.
If you forget to take Zofran melt
If you miss a dose and feel sick or vomit:
• take a Zofran melt as soon as possible, then • take your next tablet at the usual time (as shown on the label)
• do not take a double dose to make up for a forgotten dose.
If you miss a dose but do not feel sick
• take the next dose as shown on the label
• do not take a double dose to make up for a forgotten dose.
4. Possible side effects
Like all medicines, Zofran melt can cause side effects, although not everybody gets them.
Allergic reactions
If you have an allergic reaction, stop taking it and see a doctor straight away. The signs may include:
• sudden wheezing and chest pain or chest tightness
• swelling of your eyelids, face, lips, mouth or tongue
• skin rash - red spots or lumps under your skin (hives) anywhere on your body
• collapse.
Other side effects include:
Very common (may affect more than 1 in 10 people)
• headache.
Common (may affect up to 1 in 10 people)
• a feeling of warmth or flushing
• constipation
• changes to liver function test results (if you take Zofran melt with a medicine called cisplatin, otherwise this side effect is uncommon).
Uncommon (may affect up to 1 in 100 people)
• hiccups
• low blood pressure, which can make you feel faint or dizzy
• uneven heart beat
• chest pain
• fits
• unusual body movements or shaking.
Rare (may affect up to 1 in 1,000 people)
• feeling dizzy or light headed
• blurred vision
• disturbance in heart rhythm (sometimes causing a sudden loss of consciousness).
Very rare (may affect up to 1 in 10,000 people)
• poor vision or temporary loss of eyesight, which usually comes back within 20 minutes.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Zofran melt
• Keep out of the sight and reach of children.
• Do not use Zofran melt after the expiry date which is stated on the pack after ‘Exp'.
• Do not store Zofran melt above 30oC.
• Zofran melt should only be taken out of the blister immediately before taking it.
• If your doctor tells you to stop taking Zofran melt, it is important to return any which are left over to your pharmacist.
• If your Zofran melt become discoloured or show any sign of deterioration, return them to your pharmacist.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information What Zofran melt contains
The active ingredient is ondansetron. Each oral lyophilisate contains 8mg ondansetron.
The other ingredients are gelatin, mannitol, aspartame, sodium methylhydroxybenzoate, sodium propylhydroxybenzoate, strawberry flavour.
What Zofran melt looks like and contents of the pack
Zofran melt is a round white unmarked plano-convex, oral lyophilisate. Each pack contains 10 Zofran melt units.
Manufactured by
Glaxo Wellcome GmbH & Co KG, Industrie Strasse 32-36, D-23843 Bad Oldesloe, Germany.
Aspen Bad Oldesloe GmbH, Industrie Strasse 32-36, D-23843 Bad Oldesloe, Germany.
Procured from within the EU by the Product Licence holder:
MPT Pharma Ltd, Westgate Business Park, Unit 5-7 Tintagel Way, Aldridge, Walsall, WS9 8ER, UK.
Repackaged by: xxxxxxxxxxxxxxxxxxxxxxxxxxxx
Zofran melt 8mg PL: 33532/0138
Leaflet dated 6th October 2016 Leaflet coded xxxxxxxxx
Zofran® is a registered trademark of Novartis Pharma AG, Switzerland.
Package Leaflet: Information for the User
Ondansetron 8mg oral lyophilisates
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions about your illness or your medicine, ask your doctor, nurse or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
• The name of this medicine is Ondansetron 8mg oral lyophilisates but will be referred to as Ondansetron throughout the remainder of this leaflet.
What is in this leaflet:
1. What Ondansetron is and what it is used for
2. What you need to know before you take Ondansetron
3. How to take Ondansetron
4. Possible side effects
5. How to store Ondansetron
6. Contents of the pack and other information
1. What Ondansetron is and what it is used for
Ondansetron oral lyophilisates contain a medicine called ondansetron. This belongs to a group of medicines called anti-emetics. Ondansetron oral lyophilisate is a special type of Ondansetron tablet that dissolves very quickly when put on top of the tongue.
Ondansetron is used for:
• preventing nausea and vomiting caused by chemotherapy (in adults and children) or radiotherapy for cancer (adults only)
• preventing nausea and vomiting after surgery (adults only).
Ask your doctor, nurse or pharmacist if you would like any further explanation about these uses.
2. What you need to know before you take Ondansetron Do not take Ondansetron if:
• you are taking apomorphine (used to treat Parkinson's disease)
• you are allergic (hypersensitive) to ondansetron or any of the other ingredients in Ondansetron (listed in Section 6).
If you are not sure, talk to your doctor, nurse or pharmacist before taking Ondansetron.
Warnings and precautions
Check with your doctor, nurse or pharmacist before taking Ondansetron if:
• you have ever had heart problems (e.g. congestive heart failure which causes shortness of breath and swollen ankles)
• you have an uneven heart beat (arrhythmias)
• you are allergic to medicines similar to ondansetron, such as granisetron or palonosetron
• you have liver problems
• you have a blockage in your gut
• you have problems with the levels of salts in your blood, such as potassium, sodium and magnesium.
If you are not sure if any of the above apply to you, talk to your doctor, nurse or pharmacist before taking Ondansetron.
Other medicines and Ondansetron
Please tell your doctor, nurse or pharmacist if you are taking or have recently taken or might take other medicines.
This includes medicines that you buy without a prescription and herbal medicines. This is because Ondansetron can affect the way some medicines work. Also some other medicines can affect the way Ondansetron works.
In particular, tell your doctor, nurse or pharmacist if you are taking any of the following medicines:
• carbamazepine or phenytoin used to treat epilepsy
• rifampicin used to treat infections such as tuberculosis (TB)
• antibiotics such as erythromycin or ketoconazole
• anti-arrhythmic medicines used to treat an uneven heart beat
• beta-blocker medicines used to treat certain heart or eye problems, anxiety or prevent migraines
• tramadol, a pain killer.
• medicines that affect the heart (such as haloperidol or methadone)
• cancer medicines (especially anthracyclines and trastuzumab).
• SSRIs (selective serotonin reuptake inhibitors) used to treat depression and/or anxiety including fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram
• SNRIs (serotonin noradrenaline reuptake inhibitors) used to treat depression and/or anxiety including venlafaxine, duloxetine.
If you are not sure if any of the above applies to you, talk to your doctor, nurse or pharmacist before having Ondansetron.
Pregnancy and breast-feeding
It is not known if Ondansetron is safe during pregnancy. If you are pregnant, think you are pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking Ondansetron.
Do not breast-feed if you are taking Ondansetron. This is because small amounts pass into the mother's milk. Ask your doctor or midwife for advice.
Important information about some of the ingredients of Ondansetron
• This medicine contains aspartame (which is a source of phenylalanine). If you have an inherited illness called phenylketonuria speak to your doctor before taking this medicine.
• This medicine contains sodium methyl para-hydroxybenzoate and sodium propyl para-hydroxybenzoate. This may cause allergic reactions (which could be delayed).
3. How to take Ondansetron
Always take Ondansetron exactly as your doctor has told you. You should check with your doctor, nurse or pharmacist if you are not sure. The dose you have been prescribed will depend on the treatment you are having.
To prevent nausea and vomiting from chemotherapy or radiotherapy
On the day of chemotherapy or radiotherapy
• the usual adult dose is 8mg taken one or two hours before treatment and another 8mg twelve hours after.
On the following days
• the usual adult dose is 8mg twice a day
• this may be given for up to 5 days.
Children aged over 6 months and adolescents:
The doctor will decide the dose depending on the child's size (body surface area) or weight. Look at the label for more information.
• the usual dose for a child is up to 4mg twice a day
• this can be given for up to 5 days.
To prevent nausea and vomiting after an operation
The usual adult dose is 16mg before your operation
Children aged over 1 month and adolescents:
It is recommended that Ondansetron is given as an injection.
Patients with moderate or severe liver problems
The total daily dose should not be more than 8mg.

How to remove Ondansetron oral lyophilisates from the blister
and take the medicine
• Do not take an Ondansetron oral lyophilisate out from its blister until you are ready to take it.
• Before you take the Ondansetron oral lyophilisate make sure the foil packaging has not been pierced.
• Important: Do not try to push Ondansetron oral lyophilisate through the foil top like a usual tablet. This is because Ondansetron oral lyophilisate is fragile and will break.

Tear off one Ondansetron Peel back the foil, as
oral lyophilisate in its 2 shown by the arrow
blister
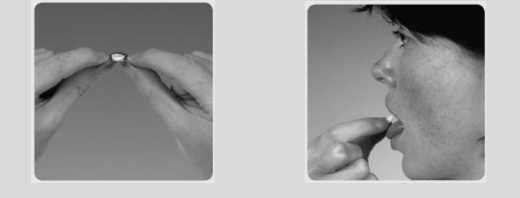
3 Gently push out the A Place the oral lyophilisate
Ondansetron oral 4 on top of the tongue.
lyophilisate. It will dissolve very quickly.
Then you can swallow as normal.
Ondansetron should start to work within one or two hours of taking a dose.
If you are sick (vomit) within one hour of taking a dose
• take the same dose again
• otherwise, do not take more Ondansetron than the label says.
If you continue to feel sick, tell your doctor or nurse.
If you take more Ondansetron than you should
If you or your child take more Ondansetron than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you.
If you forget to take Ondansetron
If you miss a dose and feel sick or vomit:
• take an Ondansetron as soon as possible, then
• take your next tablet at the usual time (as shown on the label)
• do not take a double dose to make up for a forgotten dose.
If you miss a dose but do not feel sick
• take the next dose as shown on the label
• do not take a double dose to make up for a forgotten dose.
4. Possible side effects
Like all medicines, Ondansetron can cause side effects, although not everybody gets them.
Allergic reactions
If you have an allergic reaction, stop taking it and see a doctor straight away. The signs may include:
• sudden wheezing and chest pain or chest tightness
• swelling of your eyelids, face, lips, mouth or tongue
• skin rash - red spots or lumps under your skin (hives) anywhere on your body
• collapse.
Other side effects include:
Very common (may affect more than 1 in 10 people)
• headache.
Common (may affect up to 1 in 10 people)
• a feeling of warmth or flushing
• constipation
• changes to liver function test results (if you take Ondansetron with a medicine called cisplatin, otherwise this side effect is uncommon).
Uncommon (may affect up to 1 in 100 people)
• hiccups
• low blood pressure, which can make you feel faint or dizzy
• uneven heart beat
• chest pain
• fits
• unusual body movements or shaking.
Rare (may affect up to 1 in 1,000 people)
• feeling dizzy or light headed
• blurred vision
• disturbance in heart rhythm (sometimes causing a sudden loss of consciousness).
Very rare (may affect up to 1 in 10,000 people)
• poor vision or temporary loss of eyesight, which usually comes back within 20 minutes.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Ondansetron
• Keep out of the sight and reach of children.
• Do not use Ondansetron after the expiry date which is stated on the pack after ‘Exp'.
• Do not store Ondansetron above 30oC.
• Ondansetron oral lyophilisate should only be taken out of the blister immediately before taking it.
• If your doctor tells you to stop taking Ondansetron oral lyophilisates, it is important to return any which are left over to your pharmacist.
• If your Ondansetron oral lyophilisates become discoloured or show any sign of deterioration, return them to your pharmacist.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Ondansetron contains
The active ingredient is ondansetron. Each oral lyophilisate contains 8mg ondansetron.
The other ingredients are gelatin, mannitol, aspartame, sodium methylhydroxybenzoate, sodium propylhydroxybenzoate, strawberry flavour.
What Ondansetron looks like and contents of the pack
Ondansetron oral lyophilisate is a round white unmarked plano-convex, oral lyophilisate.
Each pack contains 10 Ondansetron oral lyophilisate units.
Manufactured by
Glaxo Wellcome GmbH & Co KG, Industrie Strasse 32-36, D-23843 Bad Oldesloe, Germany.
Aspen Bad Oldesloe GmbH, Industrie Strasse 32-36, D-23843 Bad Oldesloe, Germany.
Procured from within the EU by the Product Licence holder:
MPT Pharma Ltd, Westgate Business Park, Unit 5-7 Tintagel Way, Aldridge, Walsall, WS9 8ER, UK.
Repackaged by: xxxxxxxxxxxxxxxxxxxxxxxxxxxx
Ondansetron 8mg oral lyophilisates PL: 33532/0138
Leaflet dated 6th October 2016 Leaflet coded xxxxxxxxx
POM