Zolmitriptan 5Mg Orodispersible Tablets
PACKAGE LEAFLET: INFORMATION FOR THE USER
Zolmitriptan 2.5 mg orodispersible tablets Zolmitriptan 5 mg orodispersible tablets
Zolmitriptan
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Zolmitriptan orodispersible tablets are and what they are used for
2. What you need to know before you take Zolmitriptan orodispersible tablets
3. How to take Zolmitriptan orodispersible tablets
4. Possible side effects
5. How to store Zolmitriptan orodispersible tablets
6. Contents of the pack and other information
1. What Zolmitriptan orodispersible tablets is and what it is used for
Zolmitriptan belongs to a group of medicines called “selective serotonin (5HT1) agonists” and works in your brain to relieve the symptoms of migraine.
Zolmitriptan is used for the treatment of migraine. You should not take Zolmitriptan to prevent migraines occuring.
2. What you need to know before you take Zolmitriptan orodispersible tablets
Do not take Zolmitriptan
• If you are allergic to zolmitriptan, peanut or soya or to any of the other ingredients of this medicine (listed in section 6).
• If you suffer from high blood pressure which is difficult to treat or have poorly controlled blood pressure. Speak to your doctor if unsure
• If you have had a heart attack, have heart disease, suffer from angina (chest pain) or have other circulatory problems
• If you are taking certain other medicines used for treatment or prevention of migraine such as ergotamine, dihydroergotamine, methysergide, sumatriptan or naratriptan
• If you have severe kidney failure
• If you have previously had a stroke or a transient ischaemic attack (a mini-stroke which gets completely better within a day or two)
Do not take Zolmitriptan if any of the above apply to you. If you are not sure, talk to your doctor or
pharmacist before taking Zolmitriptan.
Warnings and precautions
Talk to your doctor or pharmacist before taking Zolmitriptan if you:
• Have an irregular heart beat
• Suffer from a condition called Wolff-Parkinson-White Syndrome which is characterised by an abnormal heart rhythm.
• Smoke, suffer from high cholesterol, have diabetes, have high blood pressure, have a history of heart disease in your family or have any other condition which puts you at higher risk of developing heart disease. Your doctor may decide to conduct some additional tests to make sure that it is safe for you to take Zolmitriptan, especially if you are male and over 40 or are female and have gone through the menopause
• Are taking a herbal remedy called St. John’s wort
• Are taking any medicine for the treatment of depression such as fluoxetine, sertraline or venlafaxine. Taking Zolmitriptan together with any of these medicines may cause a life-threatening condition called Serotonin syndrome which is characterised by agitation, hallucinations, a fast heart beat, lack of coordination, vomiting, feeling sick or diarrhoea.
If you experience chest pain or chest tightness you should stop taking this medicine and contact your doctor straight away.
Zolmitriptan can lead to an increase in blood pressure. If your blood pressure rises too high you may experience symptoms such as headache, dizziness or ringing in the ears. If this applies to you you should contact your doctor.
Overuse of some common painkillers can make headaches worse. If you take common painkillers (e.g. paracetamol) regularly and suffer from frequent or daily headaches you should discuss this with your doctor.
It is not recommended to take Zolmitriptan during the aura phase (period that precedes the head pain) to prevent migraine headaches from developing. You should take your medicine during the headache phase of the migraine.
Other medicines and Zolmitriptan
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The effects of Zolmitriptan may be altered or undesirable effects may occur if you are taking other medicines. In particular, talk to your doctor if you are taking any of the following:
• Ergotamine (also used for treatment of migraine) or ergo-type medicines, such as dihydroergotamine or methysergide. You should not take Zolmitriptan within 24 hours of using ergotamine. You should not take ergotamine within 6 hours of taking Zolmitriptan
• Other triptans, such as sumatriptan or naratriptan. You should not take Zolmitriptan within 24 hours of using another triptan. You should not take another triptan within 24 hours of using Zolmitriptan
• Moclobamide, fluvoxamine, selegiline, fluoxetine, paroxetine or sertraline (for treatment of depression)
• SNRIs (serotonin norepinephrine reuptake inhibitors) such as venlaflaxine or duloxetine (for treatment of depression)
• Cimetidine (for treatment of indigestion or stomach ulcers)
• Certain antibiotics used for treatment of infection (e.g. ciprofloxacin, levofloxacin, norfloxacin or ofloxacin)
• St. John’s wort (herbal remedy containing Hypericum perforatum).
Zolmitriptan with food and drink
You can take Zolmitriptan with or without food. It does not affect the way that Zolmitriptan works. Pregnancy, breast-feeding and fertility
Pregnancy:
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Breast-feeding:
The active substance in your medicine may pass into your breastmilk. To minimise the risk of exposing your baby to your medicine you should not breast-feed for 24 hours after taking Zolmitriptan.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
During a migraine you may experience drowsiness. If affected do not drive, operate heavy machinery or participate in any other dangerous activity that requires your full attention.
Zolmitriptan contains lactose, sucrose, soya lecithin and aspartame.
Zolmitriptan contains the sugars lactose and sucrose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
Zolmitriptan also contains soya lecithin. If you are allergic to peanut, soya, do not use this medicinal product.
Zolmitriptan also contains aspartame, a source of phenylalanine. This may be harmful for people with phenylketonuria.
3. How to take Zolmitriptan orodispersible tablets
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is 2.5 mg which should be taken as soon as possible after your migraine starts. If taken later it will still work.
If a dose of 2.5 mg is not strong enough to treat your symptoms, your doctor may advise you to take a higher dose of 5 mg next time you have a migraine. You are more likely to suffer side effects with the higher dose (5 mg).
If your symptoms go away but then come back within 24 hours a second dose can be taken. However, you should wait until at least two hours after your first dose.
You do not have to take your tablet with liquid. The tablet will dissolve directly in your mouth. Put the tablet on your tongue and once it is dissolved swallow it with saliva. You should not take more than 2 doses within any 24 hour period. The maximum daily dose of your medicine is 10 mg.
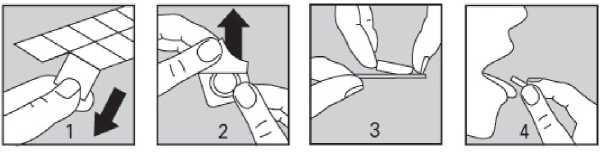
Please follow these steps to remove the tablet from the blister strip:
1. Separate one individual blister cell from the rest of the strip by gently tearing along the perforations around it
2. Peel off the backing
3. Carefully take the tablet out (do not push it out)
4. Put the tablet on your tongue, allow it to dissolve directly in your mouth and swallow it with saliva
If you have liver problems, or are taking certain other medicines your doctor may decide that you need a lower dose.
Zolmitriptan is not recommended in patients under 18 or over 65 years old.
If you take more Zolmitriptan than you should
If you (or someone else) swallow a lot of the tablets all together or if you think a child has swallowed any of the tablets, contact your nearest hospital casualty department or your doctor immediately. Please take this leaflet, any remaining tablets and the container with you to the hospital or doctor so that they know which tablets were consumed.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following side effects, stop taking Zolmitriptan and contact your doctor straight away:
Rare: may affect up to 1 in 1,000 people
• Allergic reactions including nettle rash, swelling of the face, lips, mouth, tongue, throat or difficulty in breathing.
Very rare: may affect up to 1 in 10,000 people
• Heart attack or chest pain.
• Intestinal and spleen infarction which may cause stomach pain or bloody diarrhoea.
Other possible side effects:
Common: may affect up to 1 in 10 people
• Abnormal sensations such as tingling or prickling sensation on the skin, warm sensation, increased sensitivity to touch or sound
• Dizziness or headache
• Sleepiness
• Palpitations (awareness of your heartbeat)
• Pain in the abdomen, nausea (feeling sick), vomiting, dry mouth, dysphagia (difficulties in swallowing).
• Muscle weakness and muscular pain
• Weakness
• Heaviness, tightness, pain or pressure in the throat, neck, arms and legs or chest. Uncommon: may affect up to 1 in 100 people
Fast heart beat
• Increase in blood pressure
• Need to urinate more frequently or increase in amount of urine you pass.
Very rare: may affect up to 1 in 10,000 people
• Need to urinate more urgently.
Some of the symptoms described above may be caused by your migraine itself and may not be due to your medicine.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Zolmitriptan orodispersible tablets Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Do not use this medicine if you notice visible signs of deterioration.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Zolmitriptan orodispersible tablets contain
• The active substance is zolmitriptan.
2.5 mg tablets
• Each orodispersible tablet contains 2.5 mg of zolmitriptan
5 mg tablets
• Each orodispersible tablet contains 5 mg of zolmitriptan
• The other ingredients in the tablet are lactose monohydrate, silica colloidal anhydrous, maize starch, mannitol (E421), croscarmellose sodium, citric acid anhydrous, sodium hydrogen carbonate, aspartame (E951), magnesium stearate, orange flavour (sucrose, maltodextrin, natural flavours, soya lecithin, silica colloidal anhydrous).
What Zolmitriptan orodispersible tablets look like and contents of the pack
Zolmitriptan 2.5 mg orodispersible tablets are white to off-white, round flat-face bevel edged orodispersible tablets, engraved with ‘93’ on one side and ‘8147’ on the other side.
Zolmitriptan 5 mg orodispersible tablets are white to off-white, round flat-face bevel edged orodispersible tablets, engraved with ‘93’ on one side and ‘8148’ on the other side.
Zolmitriptan orodispersible tablets are supplied in aluminium blisters containing 2, 3, 6, 12 or 18 tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
TEVA UK Limited, Eastbourne, BN22 9AG, United Kingdom
OR*
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne, BN22 9AG, United Kingdom Manufacturer:
Pharmachemie B.V., Swensweg 5, Postbus 552, 2003 RN Haarlem,The Netherlands OR*
TEVA Pharmaceutical Works Private Limited Company, Pallagi ut 13, 4042 Debrecen, Hungary OR*
TEVA Sante SA, Rue Bellocier, 89107 Sens,France OR*
Teva.Operations Poland Sp. Z.o.o., ul. Mogilska 80, 31-546 Krakow, Poland OR*
Merckle GmbH, Ludwig-Merckle-StraBe 3, 89143 Blaubeuren, Germany This leaflet was last revised in July 2014
PL 00289/1344-1345
Only the paragraph containing the details of the current batch release site will be included in the printed version of the PIL