Zomacton 10Mg/Ml Powder And Solvent For Solution For Injection In Pre-Filled Syringe
FRONT BACK

=Perforation
Pharmacode 55 mm from top and centered in box as 40 mm-
PACKAGE LEAFLET: INFORMATION FOR THE USER
ZOMACTON®
10 mg/ml, powder and solvent for solution for injection
If you develop any of the following while you are on treatment with ZOMACTON, contact your doctor or nearest casualty department at once:
• Repeated or seve re headache
• P roblems with vision
• Nausea and/or vomiting
Somatropin
Drawring
E-MS-3034
Size
510x375mm
c
o
■pH
-P
U
QJ
£_
■pH
u
Read all of this leaflet carefully before you start treatment with this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their symptoms are the same as yours.
- if you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What ZOMACTON is and what it is used for
2. What you need to know before you use ZOMACTON
3. How to use ZOMACTON
4. Possible side effects
5. How to store ZOMACTON
6. Contents of the pack and other information
In this leaflet, ‘you’ refers to you or a child or adolescent in your care.
Please consult your doctor at once if you develop a limp, or hip or knee pain.
Other medicines and ZOMACTON
Please tell your doctor or pharmacist:
• If you a re on treatment with steroids due to insufficient production of ACTH (adrenocorticotrophic hormone). This is because the dose of steroids will normally need to be adjusted while you are on treatment with ZOMACTON.
• If you a re on treatment with high dose of androgens, oestrogens or anabolic steroids as they can reduce gain in final height.
• If you a re on treatment with regularly prescribed medication e.g. steroids, medication for epilepsy or medication to suppress the body’s immune system.
• If you a re on insulin, your dose may need to be adjusted to maintain diabetic control. Your doctor will tell you if this is necessary.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
ZOMACTON must not be used during pregnancy or breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicines.
Driving and using machines
ZOMACTON has no influence on the ability to drive or use machinery.
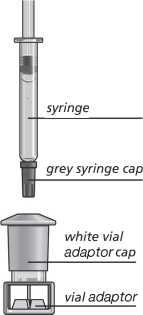
yellow cap

Zomacton 10 mg vial
ZOMACTON contains the active substance somatropin, also known as growth hormone. Growth hormone is produced naturally in the body. It has an important role in growth. ZOMACTON contains somatropin made in a pharmaceutical manufacturing facility.
ZOMACTON is used for the long-term treatment of:
• G rowth problems due to a lack of growth hormone in children;
• G rowth problems due to Turner’s syndrome (a genetic disorder affecting females).
3. HOW TO USE ZOMACTON
Always use ZOMACTON exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Your doctor or nurse will help you decide the best way for you to receive ZOMACTON.
They will also tell you the correct dose for you. The dose is given by an injection subcutaneously (under the skin) with a syringe or with the needle-free device, ZOMAJET Vision X.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ZOMACTON
Do not use ZOMACTON
• In child ren where the bone growth is completed (closed epiphyses).
• Do not use ZOMACTON and tell your doctor if you have an a ctive tumour (cancer). Tumours must be inactive and you must have finished your anti-tumour treatment before you start your treatment with ZOMACTON.
• If you a re allergic to any of the ingredients of ZOMACTON.
• If you a re seriously ill due to complications following open heart or abdominal surgery, multiple injuries from an accident, or respiratory failure.
• In child ren with chronic renal disease at time for renal transplantation.
Warnings and precautions
Talk to your doctor before using ZOMACTON.
ZOMACTON therapy should be used only under the supervision of a qualified physician experienced in
the management of patients with growth problems.
• ZOMACTON contains a p reservative called metacresol. In very rare cases the presence of metacresol can cause inflammation (swelling) in muscles. If you experience muscle pain or pain at the injection site, inform your doctor.
• Patients with Prade r-Willi syndrome should not be treated with ZOMACTON unless they are also suffering from growth hormone failure.
• If you have a family history of diabetes mellitus, you r blood sugar levels may be checked at intervals by your doctor.
• If you a re a diabetic, you will require strict monitoring of blood glucose and your dose may need to be adjusted to maintain diabetic control. Your doctor will tell you if this is necessary.
• If your growth hormone deficiency is caused by a problem in your brain (intracranial lesion), you will be carefully monitored for worsening or recurrence of this problem. If this is confirmed, the doctor will tell you if you need to stop treatment with ZOMACTON.
• If you have had a serious illness, such as cance r, treatment with ZOMACTON can make the illness return or get worse. Therefore if you notice any symptoms that worry you, you should tell your doctor immediately.
• Treatment with ZOMACTON may lead to low levels of thyroid hormone that may also need to be treated. To check for this, your doctor will normally carry out tests to ensure that your thyroid gland is working properly.
• Some child ren with growth hormone deficiency have developed leukaemia (increased number of white blood cells), whether or not they have received treatment with growth hormone. However there is no evidence that leukaemia incidence is increased in growth hormone recipients without predisposing factors. No cause and effect relationship with growth hormone treatment has been proven.
• If you a re suffering from complications following surgery, trauma or acute respiratory failure.
• If you require surgery, are seriously injured in an accident or become seriously ill, your doctor may review your treatment.
• Pancreatitis should be considered in somatropin-treated c hildren who develop abdominal pain
Dosage:
Growth hormone deficiency in children:
Your doctor will calculate the precise dose for you, based on your bodyweight in kilograms (kg). Generally, a dose of 0.17 - 0.23 mg per kg bodyweight per week is recommended. This weekly amount may be divided into six or seven doses, which means receiving a daily dose of 0.02 - 0.03 mg per kg bodyweight. The maximum recommended weekly dosage is 0.27 mg per kg bodyweight which means receiving daily injections of up to about 0.04 mg per kg bodyweight.
Turners Syndrome (females only):
Your doctor will calculate the precise dose for you, based on your bodyweight. Generally, a dose of 0.33 mg per kg bodyweight per week is recommended. This weekly amount may be divided into six or seven doses, which means receiving a daily dose of 0.05 mg per kg bodyweight.
Instructions for reconstitution
ZOMACTON is provided as a powder and should only be mixed with the solvent (liquid) provided.
The 10 mg/ml solution for injection is prepared by mixing the Zomacton powder with 1 ml of solvent using a glass syringe as described below. The steps are presented with a vial adaptor for use with the needle-free device ZomaJet Vision X and a solvent transfer connector for use for needle injections.
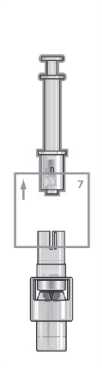
Step 7
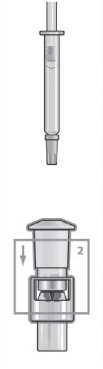
Hold the vial and firmly pull the syringe away. The syringe adaptor will remain in place.
Step 2
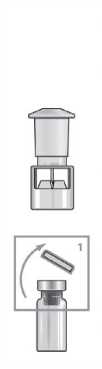
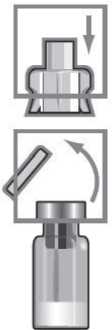
Place the vial adaptor over the centre of the vial with the spike facing downwards. Push down firmly until it clicks into place.
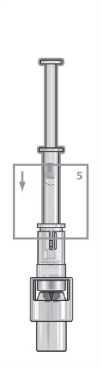
Step 5
Place the vial on a flat surface and hold the vial adaptor. Place the syringe into the vial adaptor and push down firmly


Step 8
Place the white vial adaptor cap back on the adaptor by pushing firmly until it clicks into place.
Step 9
The vial must then be swirled gently until the powder has dissolved completely to form a clear, colourless solution.
Place the reconstituted vial of ZOMACTON in an upright position in the refrigerator at 2°C to 8°C.
Avoid shaking or vigorous mixing. If the solution remains cloudy or contains particles, the vial and its contents should be discarded. In case of cloudiness after refrigeration, the solution should be allowed to warm up to room temperature. If cloudiness still persists, discard the vial and its contents.
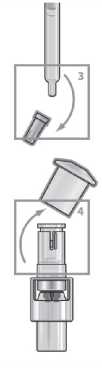
Step 2
Remove the grey syringe cap.

Step 4
Hold the vial and firmly pull the syringe away. The solvent transfer connector will remain in place.
Place the cap on the solvent transfer connector.

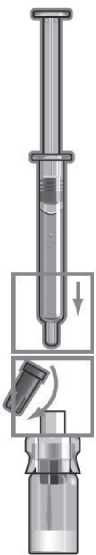
Reconstitution with solvent transfer connector for injection with an ordinary syringe
Step 1
Remove the yellow cap from the ZOMACTON vial. Place the solvent transfer connector over the centre of the vial with the spike facing downwards. Push down firmly until it clicks into place.
Step 3
Place the vial on a flat surface and hold the solvent transfer connector. Place the syringe into the solvent transfer connector and push down firmly.
Press the syringe plunger slowly. Ensure that all the solution goes into the vial.
Step 5
The vial must then be swirled gently until the powder has dissolved completely to form a clear, colourless solution.
Place the reconstituted vial of ZOMACTON in an upright position in the refrigerator at 2°C to 8°C.
Avoid shaking or vigorous mixing. If the solution remains cloudy or contains particles, the vial and its contents should be discarded. In case of cloudiness after refrigeration, the solution should be allowed to warm up to room temperature. If cloudiness still persists, discard the vial and its contents.
Administration
The required dose of ZOMACTON 10 mg/ml is administered using a needle-free ZomaJet Vision X injector or an ordinary syringe.
Specific instructions for the use of ZomaJet Vision X are given in a leaflet supplied with the device.
If you take more ZOMACTON than you should:
An overdose may cause low blood sugar (hypoglycaemia), followed by high blood sugar (hyperglycaemia).
If you or someone else has used too much ZOMACTON, contact the doctor or nearest hospital casualty department at once. The effects of repeated overdosing are unknown.
If you forget to take ZOMACTON:
In the event of a missed dose, do not worry. Carry on as usual and take the next dose at your usual time. Do not take a double dose to make up for a forgotten dose.
You may experience hypoglycaemia (low blood sugar level) which can cause dizziness, confusion and blurred vision. Although the long-term effectiveness of the treatment will not be affected, you should consult your doctor if this happens.
4. POSSIBLE SIDE EFFECTS
Like all medicines, ZOMACTON can cause side effects, although not everybody gets them.
Injecting growth hormone under the skin may lead to an increase or decrease of fat as well as punctual bleeding and bruising (purple discolouration of the skin) at the site of administration. It is therefore recommended to frequently change the site of administration. On rare occasions, patients may develop pain and an itchy rash at the site of injection.
Very commonly reported side effects (may affect more than 1 in 10 people):
Adults only:
• Swelling due to the build up of fluid, especially in the hands and feet (oedema)
• Mild high blood sugar (hype rglycaemia)
• Joint pain (arthralgia)
• Muscle pain (myalgia)
• Headache
• Numbness, tingling, bu rning or creeping on the skin (paresthesia)
Commonly reported side effects (may affect up to 1 in 10 people):
Children and adults:
• Hypothy roidism
• An immune reaction to the growth hormone, which may show up in a blood test (antibody building)
• Headache
• Inc reased tightness of muscle tone (hypertonia)
Children only:
• Swelling due to the build up of fluid, especially in the hands and feet (oedema, peripheral oedema)
• Injection site reactions
• Weakness (asthenia)
• Glucose tolerance impai red
• Joint pain (arthralgia)
• Muscle pain (myalgia)
Adults only:
• Sti ffness in the legs and/or arms
• Di fficulty falling asleep and/or difficulty staying asleep (insomnia)
Uncommonly reported side effects (may affect up to 1 in 100 people):
Children and adults:
• Anaemia
• Rapid heart rate (tachyca rdia)
• Feel a whirling or spinning (vertigo)
• Double vision (diplopia)
• Papilloedema
• Vomiting, abdominal pain, flatulence, nausea
• Weakness
• Injection site at rophy, injection site haemorrhage, injection site mass, hypertrophy
• Low blood sugar (hypoglycaemia)
• Hyperphosphatemia
• Muscle at rophy
• Bone pain
• Carpal tunnel synd rome
• Neoplasm malignant, neoplasm
• Sleepiness (somnolence)
• Involuntary eye movement (nystagmus)
• Personality diso rders
• Urinary incontinence, haematuria, polyuria, inc reased urine frequency, urine abnormality
• Injection site reactions (incl. lipodystrophy, skin atrophy, dermatitis exfoliative, urticaria, hirsutism, skin hypertrophy)
Children only:
• Stiffness in the legs and arms Adults only:
• High blood p ressure (hypertension)
Rarely reported side effects (may affect up to 1 in 1,000 people):
Children and adults:
• Diarrhoea
• Renal function test abnormal
• Diabetes mellitus type II
• Tingling or numbness in certain a reas of the body (neuropathy)
• A build up of fluid around the brain (appears as repeated or severe headache, blurred vision and nausea and/or vomiting)
Children only:
• High blood p ressure (hypertension)
• Di fficulty falling asleep, and/or difficulty staying asleep (insomnia)
• Numbness, tingling, bu rning or creeping on the skin (paresthesia)
Very rarely reported side effects (may affect up to 1 in 10,000 people):
Children only:
• Leukaemia (the occur rence appears to be no more common than in children in the general population)
• Abnormal b reasts enlargement (gynecomastia)
Reporting of side effects:
If you get any side effects, talk to your doctor or nurse. This includes any side effects not listed in this leaflet. You can also report side effects directly (see below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2.
Tel: +353 1 6764971; Fax: +353 1 6762517; Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
5. HOW TO STORE ZOMACTON
Keep this medicine out of the reach and sight of children.
Do not use ZOMACTON after the expiry date which is stated on the packaging. The expiry date refers to the last day of that month.
Store in a refrigerator at 2° C - 8° C; keep in the outer carton in order to protect it from light.
Once the powder has been dissolved in the liquid provided (reconstituted), store the vial in an upright position at 2° C - 8° C (in a refrigerator).
After mixing, the solution must be used within 28 days. You should discard any solution left in the vial at the end of this period.
If the mixture is cloudy when you take it out of the refrigerator, allow the solution to warm up to room temperature. If the mixture is still cloudy or becomes coloured, discard the vial and its contents.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. FURTHER INFORMATION
What ZOMACTON contains
The active substance is somatropin 10 mg (10 mg/ml after reconstitution).
The other ingredients are:
Powder: Mannitol, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate. Solvent: Water for injections and Metacresol.
What ZOMACTON looks like and contents of the pack
The product is a powder and solvent for solution for injection.
The powder is provided in a vial and the solvent in a syringe. The powder is white to off white in colour. When dissolved in the solvent provided, a clear, colourless solution is formed.
ZOMACTON is presented in pack sizes of 1,3 and 5 and consist of:
10 mg somatropin in vial and 1ml solvent in syringe with solvent transfer connector or vial adaptor.
Not all pack sizes may be marketed.
Marketing Authorisation Holder (UK):
Ferring Pharmaceuticals Ltd.
Drayton Hall, Church Road West Drayton UB7 7PS, UK PL 03194/0104
Marketing Authorisation Holder (Ireland):
Ferring Ireland Ltd.
United Drug House, Magna Drive Magna Business Park, Citywest Road Dublin 24, Ireland PA 1009/8/3
Manufacturer:
Ferring GmbH
Wittland 11, D-24109, Kiel
Germany