Zomacton 4Mg Injection
ZOMACT ON
Injection
Somatropin
ABOUT YOUR MEDICINE
Each Zomacton 4mg Injection carton contains a powder and solvent for solution for injection. The sterile freeze-dried powder contains the active ingredient Somatropin (Human Growth Hormone). The powder also contains the inactive ingredient mannitol. It is supplied in clear glass vials.
The solvent is supplied in an ampoule and is used to dissolve the active ingredient before it is used. The solvent contains sodium chloride and benzyl alcohol (preservative), in water for injection.
MA holder: Ferring Pharmaceuticals Ltd., Drayton Hall, Church Road, West Drayton UB7 7PS UK.
Manufacturer:
Wasserburger Arzneimittelwerk GmbH Herdestrasse 2 and Tergernau 18, 83512 Wasserburg, Germany and assembled by Ferring GmbH, Wittland 11, D-24109 Kiel, Germany.
The active ingredient, Somatropin, is the same as naturally occurring growth hormone, but is synthetically produced. This means there is no risk of developing Creutzfeldt-Jakob disease which can be caused by growth hormone extracted from infected tissue.
USES
Zomacton 4mg Injection is used for the long-term treatment of:
• children who have growth failure due to insufficient growth
hormone production;
• Short stature when present as a feature of Turner's syndrome (a genetic disorder affecting females).
BEFORE STARTING TREATMENT WITH YOUR MEDICINE
The parent/s or carer of the child must read the following questions and decide whether any of them apply to the child. If the answer to any of the questions is YES and the doctor does not know about it, tell the doctor BEFORE starting treatment with the medicine.
• Is the child less than 3 years old?
• Have you been told or do you think that the growth of the child^ bones has finished?
Is the child suffering from an acute critical illness or post
surgical complications?
Does the child have an active tumour (cancer). Tumours must be inactive and you must have finished your anti-tumour treatment before you can start your treatment with Zomacton Does the child have diabetes mellitus (sugar diabetes), or is there a family history of diabetes mellitus?
Has the child been told that he/she has a deficiency of any other hormone?
■ Patient Information
Remember
Only a doctor can prescribe this medicine. It should never be given to anyone except the person it has been prescribed for It may harm them even if they have the same symptoms.
Please read this information carefully before starting treatment with the medicine.
This leaflet does not contain the complete information, so if
you have any questions or there is anything you are unsure about, ask your doctor or pharmacist.
• Is the child taking medication for any other hormone deficiency?
• Is the child taking any other medicines that the doctor does not know about including over the counter medicines?
• Is the child allergic to any of the ingredients listed?
In case of pregnancy or breast feeding, the doctor must also be told.
The solution contains benzyl alcohol 9 mg/ml.
Due to the presence of benzyl alcohol as excipient, ZOMACTON may cause toxic reactions and allergic reactions in infants and children up to 3 years old and must not be given to premature babies or neonates.
PRECAUTIONS AND WARNINGS
If you develop any of the following while you are on treatment with Zomacton 4mg Injection, contact the doctor or nearest hospital casualty department at once:
• a limp, or hip or knee pain;
• repeated or prolonged headache;
• problems with vision;
• nausea and/or vomiting.
Some children with growth hormone deficiency have developed leukaemia (increased number of white blood cells), whether or not they have received treatment with growth hormone. However there is no evidence that leukaemia incidence is increased in growth hormone recipients without predisposing factors. No cause and effect relationship with growth hormone treatment has been proven.
Pancreatitis shouls be considered in somatropin-treated children who develop abdominal pain.
HOW TO MAKE UP THE MEDICINE period. Make sure the preparation is allowed to warm up to room
The powder should only be mixed with the solvent supplied with temperature before use. If the solution remains cloudy, discard
the vial and its contents. Do NOT use Zomacton 4mg Injection if it is past the expiry date shown on the box, vial and ampoule.
If you are unsure about the storage, ask your pharmacist. It is best to return all old and unused medication to your pharmacist for safe disposal.
PLEASE DO NOTTHROWTHIS LEAFLETAWAY UNTILTHE MEDICINE IS FINISHEDAS IT MAY BE NEEDED AGAIN.
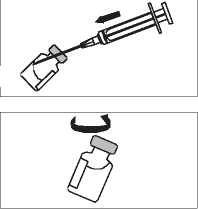
INSTRUCTIONS FOR RECONSTITUTION
la. Fit the needle onto the graduated syringe.
b. Remove the plastic top on the
vial.
null
2.
3.
FERRING
PHARMACEUTICALS
it. Use 1.3 ml of the solvent to form a solution of 3.3 mg/ml (for use with the ZomaJet 2 Vision, conventional syringe or needle device Ferring Pen). Alternatively, use 3.2 ml of the solvent to form a solution of 1.3 mg/ml (for use only with a conventional syringe). Your doctor will have told you which concentration to use. Using the syringe provided, the liquid should be injected into the vial containing the powder.To prevent foaming of the solution, inject the solvent against the wall of the vial.The vial must then be swirled gently until the powder has completely dissolved and a clear, colourless solution is produced. Avoid shaking or vigorous mixing. If the solution is cloudy or contains particles, the vial and its contents should be discarded. In case of cloudiness after refrig eration, the product should be allowed to warm up to room temperature. If cloudiness persists, discard the vial and its contents. The clear, colourless solution should then be administered as you have been shown at the clinic, using the syringe, ZomaJet 2 Vision or Ferring Pen provided for this purpose.
USINGYOUR MEDICINE
The doctor will calculate the precise dose of drug you require, according to your weight. The maximum recommended dosage is 0.27 mg per kg bodyweight, per week.
This weekly amount may be divided into seven doses with one dose each day. The dose is administered subcutaneously with a syringe, with the needle-free ZomaJet 2 Vision or with the needle device Ferring Pen. Your doctor will have given you dosage instructions and told you which method of administration to use.
In the event of a missed dose, do not worry. Carry on as usual and administer the next dose at your usual time.
OVERDOSE
An overdose may cause hypoglycaemia (low blood sugar), followed by hyperglycaemia (high blood sugar). In the event of an overdose, contact the doctor or nearest hospital casualty department at once. The effects of repeated overdosing are unknown.
SIDE EFFECTS
If you miss a dose for any reason, you may experience hypoglycaemia (low blood sugar level). Although the long-term e ec-tiveness of the treatment will not be affected, you should consult your doctor if this happens.
. Disturbances in blood sugar, including diabetes mellitus have j been observed. Very rare cases of leukaemia have been reported,
■ but it appears to be no more common than in children not treated with growth hormone.
. The subcutaneous administration of growth hormone may lead
■ to an increase or decrease of fat at the site of administration. It
" is therefore recommended to frequently change the site of administration. On rare occasions, patients develop pain and an itchy rash at the site of administration.
If you experience any incidence of blistering, bruising or abscess (redness, swelling, pain, heat, boil formation) following the use of the ZomaJet 2 Vision, please inform your doctor.
Transient headache has been reported during growth hormone replacement therapy. Rarely, a slight transient oedema (swelling due to the build up of may occur during treatment with growth hormone.
In some cases, especially during the first few weeks of therapy, repeated or prolonged headache, nausea and/or vomiting and visual problems may occur.
If these or any other undesirable effects are experienced, please inform your doctor.
STORING YOUR MEDICINE
Zomacton 4mg Injection should be stored upright in the refrigerator (2-8°C) and protected from light, both before and after making up the solution. Once the powder has been mixed with the solvent, the resulting solution must be used within 14 days. You should discard any solution left in the vial at the end of this
Snap off the top of the liquid ampoule. Remove the plastic cover on the needle. Make sure that the plunger is completely pushed in before introducing the needle into the ampoule.
Slowly draw up the required vol-J ume in the syringe. Your doctor will have told you to use either
1.3 ml or 3.2 ml:
1.3 ml for a concentration of
3.3 mg/ml (administered using a syringe, ZomaJet 2 Vision or Ferring Pen).
3.2 ml for a concentration of
1.3 mg/ml (administered using a syringe only).
To prevent foaming of the solution, inject the liquid against the sideofthe vial.
4. The vial must then be swirled gently until the powder has dissolved completely and a clear, colourless solution is produced. Avoid shaking or vigorous mixing. If the solution is cloudy or contains particles, the vial and its contents should be discarded. In case of cloudiness after refrigeration, the product should be allowed to warm up to room temperature (25°C). If cloudiness still persists, discard the vial and its contents.
The clear, colourless solution should then be administered subcutaneously as you have been shown at the clinic using the syringe, ZomaJet 2 Vision or Ferring Pen.
Pharmaceutical companies are not allowed to discuss prescription medicines with patients.
Zomacton 4mg Injection: PL 03194/0052
Isotonic Saline Solution 0.9% containing Benzyl Alcohol 0.9%:
PL 03194/0054
This leaflet was last revised in June 2013.
Zomacton and ZomaJet are Registered Trademarks.
2009050212