Airsalb Cfc-Free Inhaler 100 Microgram/Dose
SZ00000LT000
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What AirSalb is and what it is used for
2. What you need to know before you use AirSalb
3. How to use AirSalb
4. Possible side effects
5. How to store AirSalb
6. Contents of the pack and other information
The name of your medicine is AirSalb CFC-free inhaler 100 microgram/dose. It will be referred to as AirSalb throughout this leaflet.
AirSalb contains the active ingredient salbutamol. AirSalb is used to treat breathing difficulties caused by the following diseases:
• asthma
• chronic obstructive pulmonary disease (COPD) including
- chronic bronchitis
- emphysema
AirSalb is additionally used to prevent asthma symptoms caused by:
• exercise or
• triggers, such as house dust, pollen, cats, dogs and cigarette smoke
AirSalb expands the airways, allowing the air to flow more freely.
AirSalb should primarily be used to relieve symptoms rather than as a regular treatment.
AirSalb only if your doctor indicates it is clearly necessary. Do not change the dosage by yourself, but always use it according to your doctor’s recommendation.
It is unknown whether AirSalb passes into the mother’s milk. Therefore, only use AirSalb if your doctor indicates it is clearly necessary.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed. Therefore, do not drive or operate machines until you know how this medicine affects you.
AirSalb is indicated for adults, adolescent and children from 4 to 11 years.
Do not use AirSalb
• if you are allergic to salbutamol or any of the other ingredients of this medicine (listed in section 6).
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
Adults and adolescents aged 12 years and over
• Relief of attacks: 1-2 inhalations as required.
• Prevent symptoms caused by exercise or triggers: 2 inhalations 10-15 minutes beforehand.
• Maximum dosage: 8 inhalations per day.
Warnings and precautions
Talk to your doctor before using AirSalb if any of the following conditions apply to you:
• severe heart disease
• a history of heart disease, irregular heart rhythm or angina
• severe and untreated high blood pressure
• an overactive thyroid gland
• too little potassium in the blood
• enlarged artery (aneurysm)
• diabetes (extra checks of blood glucose levels are recommended when beginning treatment with AirSalb)
• tumour of the adrenal medulla (pheochromocytoma). The adrenal medulla are two glands situated above the kidneys.
Other medicines and AirSalb
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
The following medicines can influence or be influenced by AirSalb:
• heart and vascular medicines which can narrow the airways and containing active substances ending in “-ol” such as propranolol (betablockers). This may cause spasms in the lung airways.
• certain medicines to treat depression:
- monoamine oxidase inhibitors (e.g. moclobemide)
- tricyclic antidepressants, such as amitriptyline
• anaesthetics (agents that induce partial or total loss of sensation), such as halothane
• medicines against irregular heartbeat, such as digoxin
• xanthine derivatives (used to help breathing), such as theophylline
• steroids (group of hormones), such as cortisone
• diuretics (water tablets), such as furosemide
Pregnancy and breast-feeding
Limited knowledge exists regarding use during pregnancy but there also is a risk for the unborn child, if your asthma is untreated during pregnancy. Therefore, you should use
Children below (4-11 years)
• Relief of attacks: 1 inhalation as required. The dose may be increased to two inhalations if required.
• Symptoms caused by exercise or triggers:
1 inhalation, or 2 if necessary 10-15 minutes beforehand.
• Maximum dosage: 8 doses per day.
Children below 4 years of age
No recommendations can be made for dosage since the effect has not yet been determined.
Contact your doctor if the treatment is not effective enough or if you need more doses per day than usual. Never increase your dose or change the duration of use without your doctor’s approval.
Inhaler test before use:
Check that the inhaler is functioning, if you use a new inhaler or if it has not been used for 7 days or more. Remove the protective cap, shake the inhaler and spray twice into the air.
Instructions for use:
Inhale sitting or standing, wherever possible.
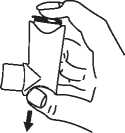
1 Remove the protective cap. Check inside and outside to make sure that the mouthpiece is clean.
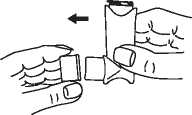
2 Shake the inhaler thoroughly for a couple of seconds before use.
00000000 Continued on the next page >>
N1
|
Artwork Proof Box | |
|
Ref: N005 - Spelling Correction | |
|
Proof no. Date prepared: |
Font size: |
|
009.0 27/05/2016 |
9pt |
|
Colours: |
Fonts: |
|
■ Black □ |
Helvetica |
|
□ □ | |
|
Dimensions: 1 50 x 460 mm |
y |
3 Hold the inhaler upright with the bottom of the container upwards, put your thumb on the base, below the mouthpiece. Breathe out as far as is comfortable, but do not breathe into the mouthpiece.
4 Place the mouthpiece in your mouth between your teeth and close your lips around it but do not bite it.
5 Just after starting to breathe in through your mouth press down on the top of the inhaler to release a puff while still breathing in steadily and deeply.
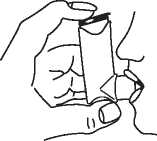
6 Hold your breath for 5-10 seconds. Take the inhaler from your mouth and your finger from the top of the inhaler.
7 If you need another puff keep the inhaler upright and wait about half a minute before repeating steps 2 to 6.
8 After use always replace the mouthpiece cover to keep out dust and fluff. Replace mouthpiece cover firmly and snap into position.
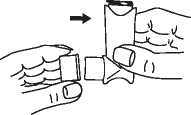
Some people find it difficult to release a puff of medicine just after they start to breathe in. In this case, as well as for children, the Vortex® or AeroChamber® Plus spacer device can be used. Please refer to the product information of the spacer device for its correct handling.
Cleaning
To prevent the inhaler blocking up or if it does block up, clean it at least once a week, as follows:
1 Pull the metal canister out of the plastic case of the inhaler and remove the mouthpiece cover.
2 Rinse the plastic case and the mouthpiece cover in lukewarm water. Do not attempt to remove any build up of medicine around the mouthpiece with a sharp object, such as a pin. A mild detergent may be added to the water, then rinse thoroughly with clean water before drying. Do not put the metal canister into water.
3 Leave the plastic case and the mouthpiece cover to dry in a warm place. Avoid excessive heat.
4 Replace the canister and mouthpiece cover. Inhaler content:
Shake the inhaler to check the remaining amount of medicine in your inhaler. Do not use AirSalb if you do not detect any liquid in the inhaler while shaking.
Cold temperature use:
If the inhaler has been stored beneath 0°C, warm it in your hands for 2 minutes, shake it and spray into the air twice before use.
If you use more AirSalb than you should
In this case always contact your doctor or hospital.
Typical symptoms of overdose are:
• trembling
• headache
• rapid heart beat
• nausea or vomiting
• inability to keep still
• irritability, excitation
• seizures
• sleepiness.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Side effects can occur with the following frequencies:
Common, may affect up to 1 in 10 people:
• trembling
• increased heart rate
• headache
• muscle cramps.
Uncommon, may affect up to 1 in 100 people:
• rapid heartbeat
• irritation in mouth and throat.
Rare, may affect up to 1 in 1,000 people:
• reduced potassium level in the blood
• flushing.
Very rare, may affect up to 1 in 10,000 people:
• allergic reactions, See "Stop using AirSalb and contact your doctor”
• decreased blood pressure
• collapse
• increased activity
• increased excitability
• hallucinations
• sleep disturbances
• irregular heart rhythm
• itchy rash
• worsening of breathing immediately after taking your inhaler.
Frequency not known (the frequency cannot be estimated from the available data):
Although it is not known exactly how often this happens, some people may occasionally experience chest pain (due to heart problems such as angina). Tell your doctor if you develop these symptoms whilst receiving treatment with AirSalb, but do not stop using this medicine unless told to do so.
Reporting of side effects If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton. The expiry date refers to the last day of that month.
Do not store above 30°C.
Store the inhaler lying flat or in an inverted position, with the mouthpiece pointing downwards.
The canister contains a pressurised liquid.
Do not expose to temperatures higher than 50°C, even for a short period of time. Protect from heat, direct sunlight and frost. Do not pierce or burn the canister even when empty.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using AirSalb and contact your doctor immediately if you develop any of the following:
• allergic reaction symptoms such as
- swelling of the face, tongue or throat
- difficulties in swallowing
- signs which resemble nettle sting
- breathing difficulties
• chest pain, see under ‘Frequency not known’ below
• breathing immediately becomes worse after taking AirSalb, although AirSalb reduces symptoms. This may mean your disease is worsening and other treatment is urgently needed.
What AirSalb contains
• The active substance is salbutamol.
One metered dose contains 100 micrograms salbutamol (as sulphate).
The delivered dose through the mouthpiece is 90 micrograms of salbutamol (as sulphate).
• The other ingredients are norflurane (HFA134a), anhydrous ethanol, oleic acid
What AirSalb looks like and contents of the pack
This medicinal product is a white pressurised inhalation suspension in aluminium containers with metering valve and plastic applicator.
Packs containing
200 metered doses equal to 8.5 g
pressurised inhalation, suspension.
2 x 200 metered doses equal to 2 x 8.5 g pressurised inhalation, suspension.
3 x 200 metered doses equal to 3 x 8.5 g pressurised inhalation, suspension.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Sandoz Ltd, Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, UK.
Manufacturer:
Aeropharm GmbH, Frangois-Mitterrand-Allee 1,07407 Rudolstadt, Germany.
+49 (0) 3672 479-0 +49 (0) 3672 479-102
This leaflet was last revised ________
00000000
in 05/2016 SZ00000LT000
/■ \
|
sg| Sag |o§ 3 0 : t 0 « C II* \L |
Artwork Proof Box Ref: N005 - Spelling Correction | |
|
Proof no. Date prepared: 009.0 27/05/2016 |
Font size: 9pt | |
|
Colours: ■ Black □ □ □ |
Fonts: Helvetica | |
|
Dimensions: 150 x 460 mm |
_J | |
|
■0 2 E | ||