Alphanine 1000 I.u. Powder And Solvent For Solution For Injection
Out of date information, search anotherGRIFOLS
PACKAGE LEAFLET: INFORMATION FOR THE USER
AlphaNine® 500 IU, 1000 IU and 1500 IU
Powder and solvent for solution for injection
Read all of this leaflet carefully before you start using this medicine because It contains Important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What AlphaNine® is and what it is used for
2. What you need to know before you use AlphaNine®
3. How to use AlphaNine®
4. Possible side effects
5. How to store AlphaNine®
6. Contents of the pack and other information
1. WHAT ALPHANINE® IS AND WHAT IT IS USED FOR
AlphaNine® is supplied as a powder and solvent for solution for injection containing 500, 1000 or 1500 IU of human coagulation factor IX per vial. (Note IU stands for international unit, a standard measure of activity).
Once reconstituted with 10 ml of water for injections (solvent), each vial contains approximately 50, 100 or 150 IU factor IX/ml.
AlphaNine® is one of the group of medicines called clotting factors.
AlphaNine® is used for the treatment and prevention of bleeding in patients with haemophilia B (congenital factor IX deficiency).
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ALPHANINE®
Do not use AlphaNine®
• If you are allergic to human coagulation factor IX or to any of the other ingredients of this medicine (listed in section 6) (please see important information about some of the ingredients of AlphaNine® at the end of this section).
• If you have not been fully trained how to inject yourself by your doctor or haemophilia nurse.
If you want more detailed information then ask your doctor.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using AlphaNine®.
• Rarely you may have an anaphylactic reaction (a sudden severe allergic reaction) such as rash, tightness of the chest, dizziness, vertigo or nausea, or feeling dizzy when you are standing. If these symptoms occur, you must stop using the product immediately and contact your doctor.
• Your doctor should perform some tests to make sure that the dosage of AlphaNine® you are receiving is enough to achieve and maintain appropriate factor IX levels and thus stop any bleeding.
• If your bleeding is not controlled with AlphaNine®, consult your doctor immediately. You may have developed factor IX inhibitors, these are antibodies which block the clotting effect of factor IX. Your doctor will perform some tests to confirm whether the inhibitors are present in your blood.
• Due to the chance of blood clots factor IX should be used with care in patients with liver disease, after surgery, in new-born infants or in patients at risk of formation of blood clots or disseminated intravascular coagulation (DIC).
Virus safety
When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include:
• careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded,
• the testing of each donation and pools of plasma for signs of virus/infections,
• the inclusion of steps in the processing of the blood or plasma that can inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus. But they may be of limited value against non-enveloped viruses such as hepatitis A virus and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (foetal infection) and for individuals whose immune system is depressed or who have some types of anaemia (e.g. sickle cell disease or haemolytic anaemia). Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly/repeatedly receive human plasma-derived factor IX products.
It is strongly recommended that every time you receive a dose of AlphaNine® the name and batch number of the product are recorded in order to maintain a record of the batches used.
See section 4.
Children and adolescents
AlphaNine® has not been studied in children under the age of 6 years so far. Therefore, AlphaNine® is not recommended to be used in children under the age of 6 years.
Other medicines and AlphaNine®
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
No interactions of AlphaNine® with other medicines are known.
Do not mix AlphaNine® with other medicines that you receive by injection.
Pregnancy and breast-feeding
Based on the rare occurrence of haemophilia B in women, experience regarding the use of factor IX during pregnancy and breast-feeding is not available.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
No effects on the ability to drive and use machines have been observed.
AlphaNine® contains sodium and heparin
AlphaNine® contains 1.3 to 3.0 mmol sodium per 10 ml (syringe). This should be taken into account by patients on a controlled sodium diet.
AlphaNine® contains small amounts of heparin. Heparin may cause allergic reactions and a reduced platelet count which may affect the blood clotting system. Patients with a history of heparin-induced allergic reactions should avoid the use of heparin-containing medicines.
3. HOW TO USE ALPHANINE®
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Reconstitute the product as described at the end of this leaflet (please see pictograms on the back of the leaflet). The product must be given by intravenous route. The administration rate should never be more than 10 ml/min to avoid undesirable side effects. Inject AlphaNine® immediately after reconstitution.
The amount of AlphaNine® you should use depends on many factors, such as your weight, your clinical status and the type and severity of bleeding. Your doctor will calculate the dose, the frequency and the intervals of administration of AlphaNine® in order to reach the necessary level of factor IX in your blood.
Your doctor will tell you the duration of your treatment with AlphaNine®.
You will be given full training before using AlphaNine® without the hospital supervision. Please refer to all your training materials or contact your local haemophilia centre for more information.
Use in children and adolescents
As with adult patients, the dosage and duration of therapy should be adjusted to the patient’s clinical condition.
If you use more AlphaNine® than you should
No cases of overdose with human coagulation factor IX have been reported. However, if you have used AlphaNine® more than required, consult your doctor or pharmacist immediately.
If you forget to use AlphaNine®
Proceed immediately with the following dose and continue at regular intervals as directed by your doctor. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, AlphaNine® can cause side effects, although not everybody gets them. On rare occasions, you may have some of the following side effects after the administration of AlphaNine®. Please contact your doctor immediately if you experience:
• itching, local reactions at the injection site (e.g. burning and transitory reddening)
• allergic reactions (e.g. tightness of the chest/feeling unwell, dizziness, nausea and slight drop of blood pressure than can make you feel dizzy when you are standing)
• fever
• faster heart beat (tachycardia)
Occasionally an anaphylactic shock may occur. If you observe any of the following symptoms during the injection/perfusion, interrupt the injection/perfusion and contact your doctor immediately:
• tightness of the chest/feeling unwell
• dizziness
• slight hypotension (slight drop of blood pressure with dizziness when you are standing)
• nausea
Allergic reactions to the components of the product cannot be totally excluded. In some cases, these reactions have progressed to severe anaphylaxis, and they have occurred at about the same time asthe development of factor IX inhibitors.The formation of neutralising antibodies of factor IX (inhibitors) is a well-known complication in the treatment of patients with haemophilia B. Study results show that first time users of factor IX are mainly affected. You will be carefully monitored for the development of these inhibitors.
Nephrotic syndrome has been reported following attempted immune tolerance induction in haemophilia B patients with factor IX inhibitors and history of allergic reactions.
There is a chance of blood clots, particularly if you have known clinical risk factors.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme. Website: https://yellowcard.mhra.gov.uk. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ALPHANINE®
Keep this medicine out of the sight and reach of children.
Store in a refrigerator (2 - 8 °C). Do not freeze. Protect from light.
The product may be removed from the refrigerator for one single period of maximum 1 month at room temperature (do not store above 30 °C).
Do not use this medicine after expiry date which is stated on the vial label and carton after EXP. Do not use this medicine if you notice the solution is cloudy or has deposits. Generally the solution is clear or slightly opalescent. If the solution is discoloured or cloudy discard it. After reconstitution with the water for injections provided the product should be used immediately.
Any unused product or waste material should be disposed of in accordance with local requirements.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
What AlphaNine® contains
The active substance is human coagulation factor IX.
AlphaNine® is presented as powder for solution for injection containing 500, 1000 or 1500 IU human coagulation factor IX per vial. The product is reconstituted with 10 ml of water for injections.
The other ingredients are heparin, dextrose, sodium hydroxide and hydrochloric acid (see section 2. Before you use AlphaNine®, for further information about ingredients).
What AlphaNine® looks like and contents of the pack
Vial containing white or pale yellow powder and syringe with water for injections (solvent). Presentations:
AlphaNine® 500 IU AlphaNine® 1000 IU AlphaNine® 1500 IU Water for injections
PL 12930/0012 PL 12930/0013 PL 12930/0014 PL 4447/0016
Pack size: 1 lyophilised vial, 1 syringe pre-filled with solvent and accessories (vial adaptor, filter, 2 alcohol swabs and butterfly needle).
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder and Manufacturer Instituto Grifols, S.A.
Can Guasc, 2 - Parets del Valles 08150 Barcelona - SPAIN Distributed by:
Grifols UK Ltd.
Byron House
Cambridge Business Park Cambridge CB4 0WZ
This leaflet was last revised in May 2015 Instructions for use/handling
Follow these instructions unless otherwise indicated by your doctor.
Left-over product must never be kept for later use, nor stored in a refrigerator.
To prepare the solution:
1. Warm the vial and syringe but not above 30 °C.
2. Attach plunger to syringe containing solvent.
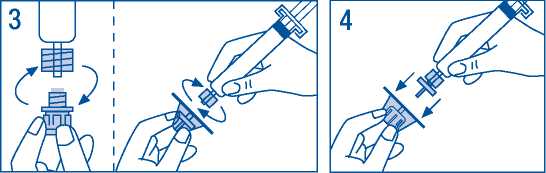
3. Remove filter from packaging. Remove cap from syringe tip and attach syringe to filter.
4. Remove vial adaptor from packaging and attach to syringe and filter.
5. Remove cap from vial and wipe stopper with swabs provided.
6. Pierce vial stopper with adaptor needle.
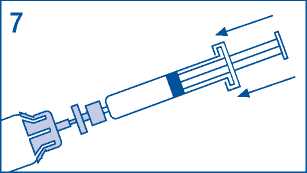
7. Transfer all solvent from syringe to vial.
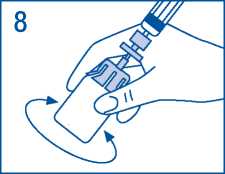
8. Gently shake vial until all product is dissolved. As with other parenteral solutions, do not use if product is not properly dissolved or particles are visible.
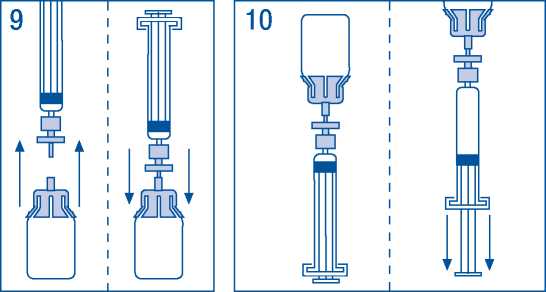
9. Briefly separate the syringe/filter from vial/adaptor, to release the vacuum.
10. Turn the vial upside down and draw the solution into the syringe.
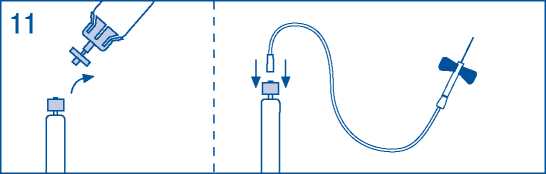
11. Prepare injection site, separate syringe and inject product using the butterfly needle provided. Injection rate should never be more than 10 ml/min to avoid vasomotor reactions.
Do not re-use administration sets.
i
A
30°C 86°F
|
2 - |
1 *4 * A" |
|
-1 |
J M |
5
A
A

A
|
6 |
iii |
|
V s_ |
P-10