Aminoplasmal Paediatric 10% Solution For Infusion
488/12614903/1115
Package leaflet: Information for the patient
= Aminoplasmal Paediatric 10% solution for infusion
For use in children (0-11 years old)
What is in this leaflet
1. What Aminoplasmal Paediatric 10% is and what it is used for
2. What you need to know before your child receives Aminoplasmal Paediatric 10%
3. How to use Aminoplasmal Paediatric 10%
4. Possible side effects
5. How to store Aminoplasmal Paediatric 10%
6. Contents of the pack and other information
6. Contents of the pack and other information
schwarz
Format = 210 x 594 mm 2 Seiten
Latus 303

GB_488
488/12614903/1115 GIF (Monobag)
Standort Melsungen (LIFE N)
Font size: 9,0 pt.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or nurse.
• This medicine has been prescribed for your child only. Do not pass it on to others. It may harm them, even if their signs of illnessare the same as your child's.
• If your child gets any side effects , talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
|
Approval for Printing B|BRAUN Melsungen AG | |
|
Approved for Printing |
□ |
|
Approved for Printing when corrected |
□ |
|
New draft required |
□ |
|
Date |
Signature |
|
Name in capital letters | |
1. What Aminoplasmal Paediatric 10% is and what it is used for_
Aminoplasmal Paediatric 10% contains amino acids that are essential for the body to grow or to recover. The solution is adapted to meet the specific needs of preterm and term newborn infants, infants and toddlers and children. They will receive this solution as part of intravenous feeding if they cannot eat food normally. They may also receive other nutrients like glucose solutions or fat emulsions in combination with Aminoplasmal Paediatric 10%.
2. What you need to know before your child receives Aminoplasmal Paediatric 10 %_
Do not use Aminoplasmal Paediatric 10%, if your child
• is allergic to active substances or any of the other ingredients of this medicine (listed in section 6)
• has inborn disorders of its protein and/or amino acid metabolism
• has a life-threatening circulatory condition (shock)
• has abnormally low levels of potassium
• has acidic substances that accumulate in blood (acidosis)
• has a severe heart weakness with impaired circulation (decompensated cardiac insufficiency)
• has an insufficient oxygen supply
• has excess body water, swelling of limbs (hyperhydration).
• an accumulation of fluid in lungs (lung oedema)
Your doctor will also take into account that amino acid containing solutions in general must not be used if your child
• suffers from a severe liver disease (severe hepatic insuffiency)
• suffers from a severe kidney failure (severe renal insufficiency) not treated with renal replacement therapy
• is in a state of coma as a result of a severe liver failure (hepatic coma)
Warnings and precautions
Talk to your doctor or nurse before you use Aminoplasmal Paediatric 10%, if your child
• suffers from an impairment of metabolism of protein and/or amino acids caused by any condition other than an inborn metabolism disorder (see section "Do not use Aminoplassmal Paediatric 10% if your child...").
• suffers from an impairment ofliver and/or kidney function
• has a high concentrated blood serum (high serum osmolarity)
• needs to restrict intake of the fluids Additional precautions taken by your doctor
Before and while children are receiving this solution, the doctor will check levels of fluids, blood chemistry and other blood levels, liver and kidney function.
Usually children will receive Aminoplasmal Paediatric 10% as part of intravenous feeding which also includes energy supplements (glucose solutions, fat emulsions), vitamins, electrolytes and trace elements.
Other medicines and Aminoplasmal Paediatric 10%
Tell your doctor, if your child is taking, has recently taken or might take any other medicines.
Pregnancy and breast-feeding
Ask your doctor for advice before you receive this medicine. Aminoplasmal Paediatric 10% is intended for use in children (under twelve years old) only.
Driving and using machines
Not relevant
What Aminoplasmal Paediatric 10% contains
The active substances are
|
Amino acids |
per 1 ml |
per 100 ml |
per 250 ml |
|
Isoleucine |
5.10 mg |
0.51 g |
1.28 g |
|
Leucine |
7.60 mg |
0.76 g |
1.90 g |
|
Lysine monohydrate (equivalent to lysine) |
9.88 mg (8.80 mg) |
0.99 g (0.88 g) |
2.47 g (2.20 g) |
|
Methionine |
2.00 mg |
0.20 g |
0.50 g |
|
Phenylalanine |
3.10 mg |
0.31 g |
0.78 g |
|
Threonine |
5.10 mg |
0.51 g |
1.28 g |
|
Tryptophan |
4.00 mg |
0.40 g |
1.00 g |
|
Valine |
6.10 mg |
0.61 g |
1.53 g |
|
Arginine |
9.10 mg |
0.91 g |
2.28 g |
|
Histidine |
4.60 mg |
0.46 g |
1.15 g |
|
Alanine |
15.90 mg |
1.59 g |
3.98 g |
|
Glycine |
2.00 mg |
0.20 g |
0.50 g |
|
Aspartic acid |
6.60 mg |
0.66 g |
1.65 g |
|
Glutamic acid |
9.30 mg |
0.93 g |
2.33 g |
|
Proline |
6.10 mg |
0.61 g |
1.53 g |
|
Serine |
2.00 mg |
0.20 g |
0.50 g |
|
N-Acetyltyrosine (equivalent to tyrosine) |
1.30 mg (1.06 mg) |
0.13 g (0.11 g) |
0.33 g (0.27 g) |
|
Acetylcysteine (equivalent to cysteine) |
0.700 mg (0.520 mg) |
0.070 g (0.052 g) |
0.175 g (0.13 g) |
|
Taurine |
0.300 mg |
0.030 g |
0.075 g |
|
per 1 ml |
per 100 ml |
per 250 ml | |
|
Amino acid content |
0.1 g |
10 g |
25 g |
|
Nitrogen content |
0.0152 g |
1.52 g |
3.8 g |
|
Energy [kJ/l (kcal/l)] |
1700 (400) | ||
|
Theoretical osmolarity [mOsm/l] |
790 | ||
|
Acidity (titration to pH 7.4) |
23 mmol | ||
|
pH |
approx. 6.1 | ||
The other ingredients are citric acid monohydrate (for pH-adjustment) and water for injection.
What Aminoplasmal Paediatric 10% looks like and contents of the pack
Aminoplasmal Paediatric 10% is a clear, colourless up to light yellow solution.
It is supplied in flexible bags containing 100 ml or 250 ml of amino acids solution. The bags are made of multilayer foil. The inner layer in contact with the solution consists of polypropylene.
Container is PVC-free, DEHP-free, latex-free.
The bag is packed in a protective plastic bag. An oxygen absorber (containing powdered iron hydroxide) is placed between bag and overwrap.
The different container sizes are presented in cartons containing 12 bags. Pack sizes: 12 x 100 ml and 12 x 250 ml Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
B. Braun Melsungen AG
Carl-Braun-StraBe 1 Postal address:
34212 Melsungen, Germany 34209 Melsungen, Germany
Phone: +49/5661/71-0 Fax: +49/5661/71-4567
3. How to use Aminoplasmal Paediatric 10%_
Aminoplasmal Paediatric 10% is given by health care professionals.
The doctor will decide how much of this medicine is needed and for how long this medicine will be given to children.
The solution will be given through a small plastic tube inserted into a vein.
If your child receives more Aminoplasmal Paediatric 10% than it should
If this medicine is given too rapidly, it may not be tolerated well. Then your child may feel sick, may have to vomit or a redening of the face and sensations of warmth might be seen. Your child may also lose amino acids in the urine and as a result from that, shifts in the proportion of amino acids in its blood may occur.
In case of an overdose your doctor will give your child any necessary treatment.
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria Aminoplasmal Paed 10% Infusionslosung
Belgium Aminoplasmal Paediatric oplossing voor infusie/solution
pour perfusion/Infusionslosung Bulgaria Aminoplasmal Paed 10%
Czech Republic Amiped Denmark Amiped
Finland Amiped
Germany Aminoplasmal Paed 10% Infusionslosung
Greece Aminoplasmal Paed 10%
Hungary Aminoplasmal Paed 10% Oldatos infuzio
Italy Amiped
Luxembourg Aminoplasmal Paed 10%
Netherlands Aminoplasmal Paed 100 mg/ml, oplossing voor infusie
Norway Amiped
Poland Aminoplasmal Paed 10%
Portugal Aminoplasmal Paed
Slovak Republic Amiped
Slovenia Aminoplasmal Paed 100 mg/ml raztopina za infundiranje
Sweden Amiped
United Kingdom Aminoplasmal Paediatric 10% solution for infusion This leaflet was last revised in September 2015.
4. Possible side effects_
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are not specifically related to the solution Aminoplasmal Paediatric 10 % but may occur with any kind of intravenous feeding, especially at the beginning of therapy.
Uncommon (may affect up to 1 in 100 people):
• feeling sick, vomiting
• headache
• shivering
• fever
Vein inflammation plus blood clotting (thrombophlebitis) may occur when the product is not sufficiently diluted and given through a peripheral vein.
Reporting of side effects
If your child gets any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Aminoplasmal Paediatric 10%_
Keep this medicine out of the sight and reach of children.
Do not freeze. Do not use if accidentally frozen and dispose of the bag. Do not use this medicine after the expiry date that is stated on the label after EXP.
The following information is intended for healthcare professionals only.
Posology
Paediatric population
The dosages for these age groups as stated below are average values for guidance. The exact dosage should be adjusted individually according to age, developmental stage, prevailing disease and type of therapy.
Dosing should be commenced below target infusion rate value and be increased to target value within the first hour.
Parenteral amino acid supply considered adequate for most paediatric patients:
Preterm infants:
1.5 - 4.0 g amino acids/kg body weight per day = 15 - 40 ml /kg b. w./d Term newborn infants (0 - 27 days):
1.5 - 3.0 g /kg b. w./d = 15 - 30 ml/kg b. w./d Infants and toddlers (28 days to 23 months):
1.0 - 2.5 g /kg b. w./d = 10 - 25 ml/kg b. w./d
Children (2 to 11 years): 1.0 - 2.0 g/kg b. w./d = 10 - 20ml/kg b. w./d For critically ill children the advisable amino acid intake may be higher (up to 3.0 g amino acids/kg body weight per day).
Maximum rate of infusion up to 0.1 g amino acids/kg body weight and hour, corresponding approximately 1 ml/kg body weight and hour
Special precautions for disposal and other handling
Do not use if overwrap has been previously opened or damaged. Only use bags that are undamaged and in which the amino acid solution is clear and free of particles.

Aminoplasmal Paediatric 10% is supplied in single use containers. Unused m residues must be discarded.
The solution should always be brought to room temperature prior to infusion.
It is essential that any admixture be prepared using strict aseptic techniques as this nutrient mixture supports microbial growth. Aminoplasmal Paediatric 10% should be used immediately after opening the container.
Aminoplasmal Paediatric 10%: Handling Figure A: Bag and Overwrap
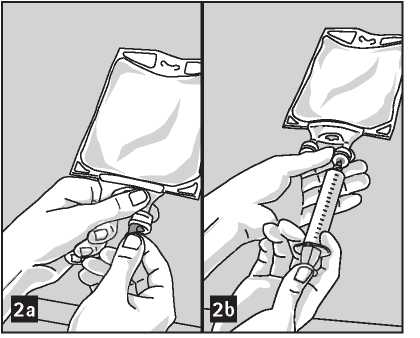
To Add Medication:
Admixtures must be prepared following strict aseptic techniques .
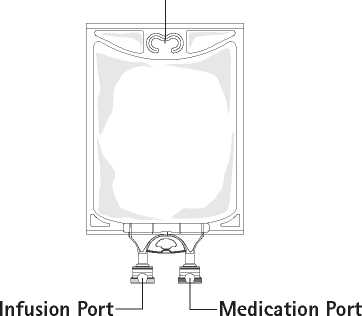
Compatible supplemental medication may be added through the
medication port (transparent colour).
1. Prepare medication port (transparent colour) by removal of aluminum foil (figure 2a). Please note: The area underneath the foil of the medication port is sterile.
2. Puncture resealable medication port and inject additive(s) (figure 2b).
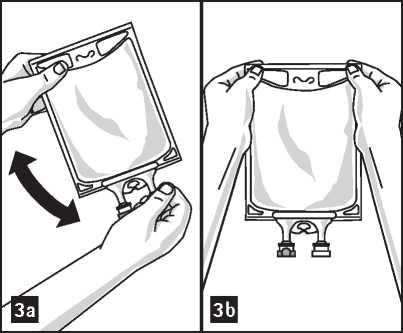
3. Mix solution and medication thoroughly (figure 3a).
4. Medication port may be swabbed with disinfection agent (e.g. isopropanol) before re-puncturing.
5. Check admixture visually for particulate matter (figure 3b).
Tear Notches Overwrap
Oxygen Absorber
Figure B: Bag
Bag Hanger
To Open:
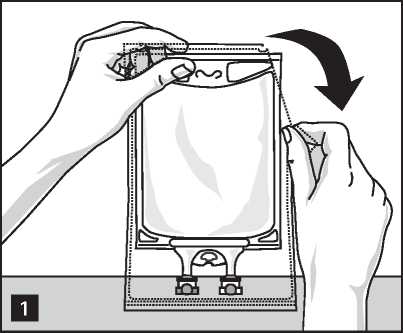
Remove the bag from its protective overwrap starting from the tear notches on top and remove solution container (figure 1).
Discard overwrap and oxygen absorber.
Check for leakages. If bag leaks, discard product as sterility may be impaired.
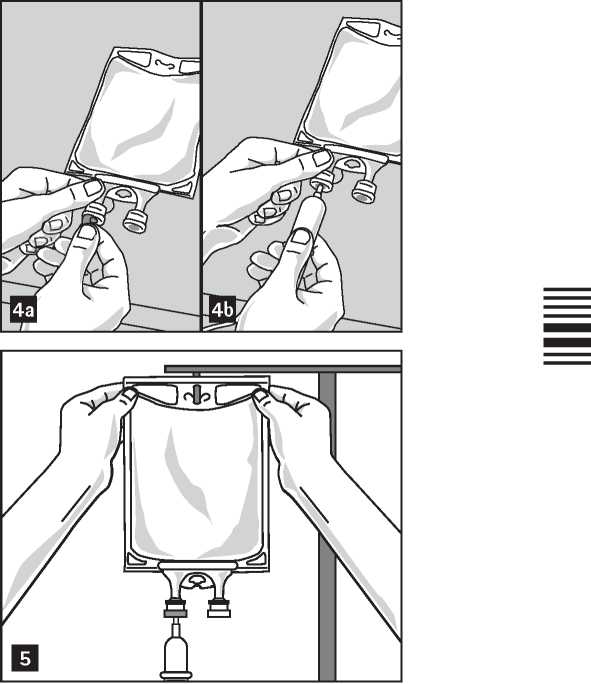
Preparation for Administration:
1. Remove aluminum foil of infusion port (green colour) at the bottom of container (figure 4a) and attach administration set (figure 4b): use non-vented infusion set or close air vent on a vented set. Follow directions for use of the infusion set. Please note: The area underneath the foil of the infusion port is sterile.
2. Hang bag on an i.v. pole (figure 5).

12614903_AminoplasmalPaed10_GIF-210x594_GB.indd 2 18.11.15 08:11