Aminoplasmal Paediatric 10% Solution For Infusion
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Aminoplasmal Paediatric 10% solution for infusion
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The solution for infusion contains:
|
Amino acids: |
per 1 ml |
per 100 ml |
per 250 ml |
|
Isoleucine |
5.10 mg |
0.51 g |
1.28 g |
|
Leucine |
7.60 mg |
0.76 g |
1.90 g |
|
Lysine monohydrate (equivalent to lysine) |
9.88 mg (8.80 mg) |
0.99 g (0.88 g) |
2.47 g (2.20 g) |
|
Methionine |
2.00 mg |
0.20 g |
0.50 g |
|
Phenylalanine |
3.10 mg |
0.31 g |
0.78 g |
|
Threonine |
5.10 mg |
0.51 g |
1.28 g |
|
Tryptophan |
4.00 mg |
0.40 g |
1.00 g |
|
Valine |
6.10 mg |
0.61 g |
1.53 g |
|
Arginine |
9.10 mg |
0.91 g |
2.28 g |
|
Histidine |
4.60 mg |
0.46 g |
1.15 g |
|
Alanine |
15.90 mg |
1.59 g |
3.98 g |
|
Glycine |
2.00 mg |
0.20 g |
0.50 g |
|
Aspartic acid |
6.60 mg |
0.66 g |
1.65 g |
|
Glutamic acid |
9.30 mg |
0.93 g |
2.33 g |
|
Proline |
6.10 mg |
0.61 g |
1.53 g |
|
Serine |
2.00 mg |
0.20 g |
0.50 g |
|
N-Acetyltyrosine (equivalent to tyrosine) |
1.30 mg (1.06 mg) |
0.13 g (0.11 g) |
0.33 g (0.27 g) |
|
Acetylcysteine (equivalent to cysteine) |
0.700 mg (0.520 mg) |
0.070 g (0.052 g) |
0.175 g (0.13 g) |
|
Taurine |
0.300 mg |
0.030 g |
0.075 g |
|
per 1 ml |
per 100 ml |
per 250 ml | |
|
Amino acid content |
0.1 g |
10 g |
25 g |
|
Nitrogen content |
0.0152 g |
1.52 g |
3.8 g |
For the full list of excipients, see section 6.1.
PHARMACEUTICAL FORM
3
Solution for infusion
Clear, colourless up to light yellow solution
|
Energy [kJ/l (kcal/l)] |
1700 (400) |
|
Theoretical osmolarity [mOsm/l] |
790 |
|
Acidity (titration to pH 7.4) |
23 mmol |
|
pH |
approx. 6.1 |
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Supply of amino acids as part of parenteral nutrition in combination with energy (glucose and lipids) and electrolytes containing solutions for newborn infants, term and preterm, infants and toddlers and children, when oral or enteral nutrition is impossible, insufficient or contraindicated.
4.2 Posology and method of administration
Posology
Paediatric population
The dosages for these age groups as stated below are average values for guidance. The exact dosage should be adjusted individually according to age, developmental stage, prevailing disease and type of therapy.
Dosing should be commenced below target infusion rate value and be increased to target value within the first hour.
Parenteral amino acid supply considered adequate for most paediatric patients: Preterm infants: 1.5 - 4.0g amino acids/kg body weight per day =15 -40ml/kg b. w./d
Term newborn infants (0 - 27 days): 1.5 - 3.0g/kg b. w./d = 15 - 30ml/kg b. w./d.
Infants and toddlers (28 days to 23 months): 1.0 - 2.5g/kg b. w./d = 10 -25ml/kg b. w./d
Children (2 to 11 years): 1.0 - 2.0g/kg b. w./d = 10 - 20ml/kg b. w./d
For critically ill children the advisable amino acid intake may be higher (up to 3.0g amino acids/kg body weight per day).
Maximum rate of infusion up to 0.1g amino acids/kg body weight and hour, corresponding approximately 1ml/kg body weight and hour
Method of administration Intravenous use.
Due to its high osmolarity, undiluted Aminoplasmal Paediatric 10% should only be administered through a central vein. However, sufficient dilution of
Aminoplasmal Paediatric 10% for example with water for injection or a suitable solution lowers the osmolarity and allows peripheral infusion.
If concomitant supplies of energy and electrolytes are adequate, there are no restrictions as part of a parenteral nutritional regimen.
Aminoplasmal Paediatric 10% merely represents a basis for parenteral nutrition. In patients on parenteral nutrition alone, concomitant replacement with energy sources (taking essential fatty acid requirements into account), electrolytes, vitamins and trace elements is required.
4.3
Contraindications
• Hypersensitivity to the active substance(s) or to any of the excipients listed in section 6.1
• States of hyperhydration, hypokalaemia
• Inborn errors of amino acid metabolism
• Acute metabolic disturbances due to hypoxia and acidosis
• Life-threatening circulatory instability
• Acute pulmonary oedema
• Decompensated cardiac insufficiency
As for all amino acid containing solutions, contraindications for Aminoplasmal Paediatric 10% are severe hepatic insufficiency, hepatic coma and severe renal insufficiency in the absence of renal replacement therapy.
4.4 Special warnings and precautions for use
Caution should be exercised if serum osmolarity is increased.
Caution should be exercised if fluid intake has to be restricted, such as in the presence of hyponatraemia.
In particular, more frequent monitoring of clinical state and appropriate laboratory values is required in patients with:
• Disturbances of amino acid metabolism.
• Hepatic impairment, due to the risk of recurrence or exacerbation of existing neurological disorders associated with hyperammonaemia.
• Renal insufficiency, particularly in the presence of hyperkalaemia, or risk factors for the development or exacerbation of metabolic acidosis, or with hyperazotaemia due to impaired renal clearance.
The dosage should be adjusted to age, nutritional status and the underlying disease. See section 4.2.
Furthermore, it is essential that therapy be supplemented by providing adjuvant sources of energy (glucose and lipids), vitamins and trace elements.
Paediatric formulations should be used in the case of micronutrients.
Before Aminoplasmal Paediatric 10% is administered via a peripheral vein, it must be sufficiently diluted with a suitable solution. See section 4.2.
Fluid and electrolyte balance, serum osmolarity, acid-base balance, blood glucose levels, renal function and liver values should be monitored throughout the duration of parenteral therapy, with the frequency of monitoring being determined by the severity of the underlying disease and the patient’s clinical status.
Determination of serum urea nitrogen and serum ammonia concentrations should be performed.
During long-term use (several weeks), the blood count and coagulation factors should be monitored more carefully.
4.5 Interaction with other medicinal products and other forms of interaction
None known
4.6 Fertility, pregnancy and lactation
Aminoplasmal Paediatric 10% is intended for use in the paediatric population only.
4.7 Effects on ability to drive and use machines
Not relevant
4.8 Undesirable effects
Undesirable effects that, however, are not specifically related to the product but to parenteral nutrition in general may occur, especially at the beginning of parenteral nutrition.
Uncommon (>1/1,000 to <1/100):
Gastrointestinal disorders: Nausea, vomiting General disorders: Headache, shivering, fever
Thrombophlebitis can occur when the product is administered through a peripheral vein if not sufficiently diluted, due to the high osmolarity of the solution.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse via the MHRA Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
4.9 Overdose
Symptoms
Too high infusion rates may lead to intolerance reactions manifesting in the form of nausea, vomiting, flushing of head, sensation of warmth, renal amino acid losses with consecutive amino acid imbalances.
Treatment
If intolerance reactions occur, the amino acid infusion should be interrupted temporarily and later on resumed at a lower infusion rate.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Solutions for parenteral nutrition, amino acids ATC code: B05BA01
Mechanism of action
The solution for infusion contains 19 amino acids to cover the child’s qualitative protein requirements:
- all amino acids essential and semi-essential for children
Compared to conventional amino acid solutions Aminoplasmal Paediatric 10% contains:
- a relatively high proportion of lysine
- taurine
- a relatively low proportion of methionine
- a low proportion of phenylalanine and proline.
The solutions contain no additional electrolytes, in order to exclude any effect on electrolyte treatment in children during resuscitation.
Pharmacodynamic effect
Amino acids represent the building blocks for protein synthesis. Pure amino acid solutions are administered as part of parenteral nutrition therapy together with sources of energy and other nutrients such as electrolytes, trace elements, vitamins and fluids, in order to maintain and improve the organism’s nutritional status or to minimise any loss of substances.
5.2 Pharmacokinetic properties
Absorption
Aminoplasmal Paediatric 10% is infused intravenously. Hence, all substrates are available for metabolism immediately.
Distribution
Free amino acid concentrations in plasma are subject to considerable fluctuations. This applies both to individual amino acids and the sum of amino acid concentrations. In contrast, amino acid interrelationships remain relatively constant, regardless of the total amino acid concentration or absolute concentration levels of the individual amino acids. It would appear that the organism strives to maintain the “amino acid” substrate at a constant level within a physiological reference range and to avoid imbalances in the amino acid pattern as much as possible. In most cases where the organism’s possibilities for compensation are maintained, drastic changes in substrate administration lead only to impaired amino acid homeostasis in the blood. Typical pathological changes in the plasma amino acid profile are expected only when the regulatory index of basic metabolic organs, e.g. liver or kidney, is considerably impaired. If necessary, such changes can be treated, in terms of restoring homeostasis, with amino acid solutions with a specific composition.
Under pathological conditions without exogenous supply of amino acids, considerable typical changes in the plasma amino acid pattern occur, generally affecting both the absolute concentration of individual amino acids and their percentage composition in plasma.
Biotransformation
Amino acids as a whole are a complex system of substances that mutually influence each other. On the one hand, there is a direct metabolic dependency between individual amino acids (for example: production of tyrosine via hydroxylation of phenylalanine). On the other hand, other metabolic mechanisms in the organism may show a sensitive response to a shift in the amino acid pattern, by altering the concentration of individual amino acids or groups of amino acids (for example: changes in the ratio of aromatic to branched-chain amino acids). In addition, changes in relationships within a group of amino acids with a similar chemical configuration and similar metabolic behaviour may have effects on the organism’s overall metabolism.
5.3
Preclinical safety data
Non-clinical studies have not been performed with Aminoplasmal Paediatric 10%.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Citric acid monohydrate (for pH-adjustment) Water for injection
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.3.
6.3 Shelf life
- Unopened (bag in overwrap) :
2 years
- After first opening:
Product should be used immediately.
- After mixing with other components of parenteral nutrition:
Aminoplasmal Paediatric 10% should only be mixed with other iv solutions, if compatibility has been proven in advance.
Compatibility data for different additives (e.g. glucose, lipids, electrolytes, trace elements, vitamins) and the corresponding shelf-life of such admixtures can be provided on demand by the manufacturer.
Chemical and physical in-use stability after mixture with those solutions has been demonstrated for 24 hours at 2-8°C plus 24 hours at 37°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
6.4 Special precautions for storage
Do not freeze. If accidentally frozen, discard the bag.
For storage conditions after admixture of additives see section 6.3.
6.5 Nature and contents of container
Aminoplasmal Paediatric 10% is supplied in flexible bags made from a multilayer foil (Polypropylene, Styrene Ethylene Butylene Styrene (SEBS) and Copolyester ether). The inner layer in contact with the solution consists of polypropylene. The bags contain 100ml or 250ml.
The bag is packed in a protective overwrap. An oxygen absorber (sachet of inert material contains powdered iron hydroxide) is placed between the bag and the overwrap.
The different container sizes are presented in cartons containing 12 bags. Pack sizes: 12 x 100ml and 12 x 250ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements are needed for disposal of container, overwrap and oxygen absorber.
Do not use if overwrap has been previously opened or damaged. Only use bags that are undamaged and in which the amino acid solution is clear and free of particles.
Aminoplasmal Paediatric 10% is supplied in single use containers. Unused residues must be discarded.
The solution should always be brought to room temperature prior to infusion.
It is essential that any admixture be prepared using strict aseptic techniques as this nutrient mixture supports microbial growth.
Aminoplasmal Paediatric 10%: Handling Figure A: Bag and Overwrap
To Open:
Remove the bag from its protective overwrap starting from the tear notches on top and remove solution container (figure 1).
Discard overwrap and oxygen absorber.
Check for leakages. If bag leaks, discard product as sterility may be impaired.
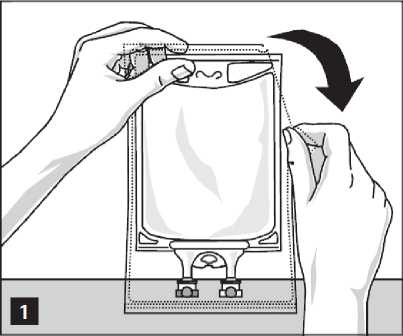
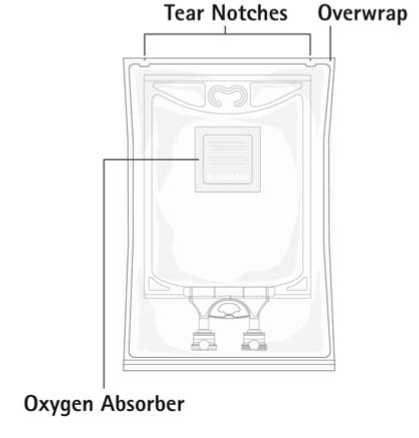
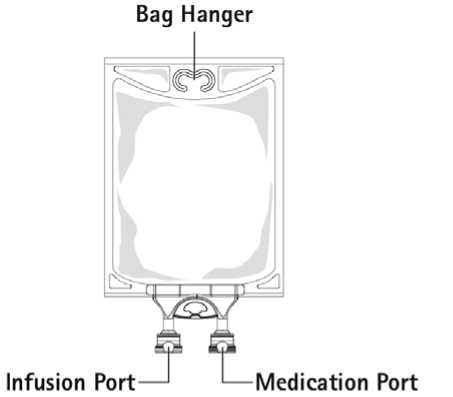
Figure B: Bag
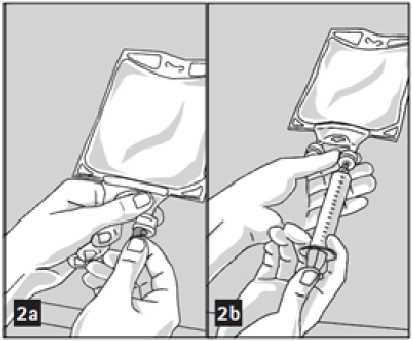
To Add Medication:
Admixtures must be prepared following strict aseptic techniques.
Compatible supplemental medication may be added through the medication port (transparent colour).
1. Prepare medication port (transparent colour) by removal of aluminium foil (figure 2a). Please note: The area underneath the foil of the medication port is sterile.
2. Puncture resealable medication port and inject additive(s) (figure 2b).
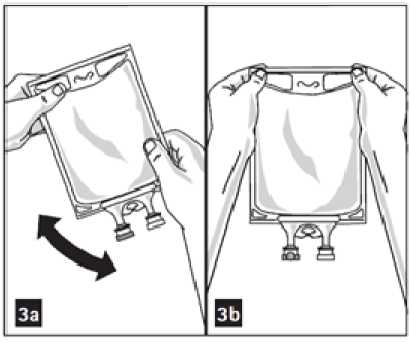
3. Mix solution and medication thoroughly (figure 3a).
4. Medication port may be swabbed with disinfection agent (e.g. isopropanol) before re-puncturing.
5. Check admixture visually for particulate matter (figure 3b).
Preparation for Administration:
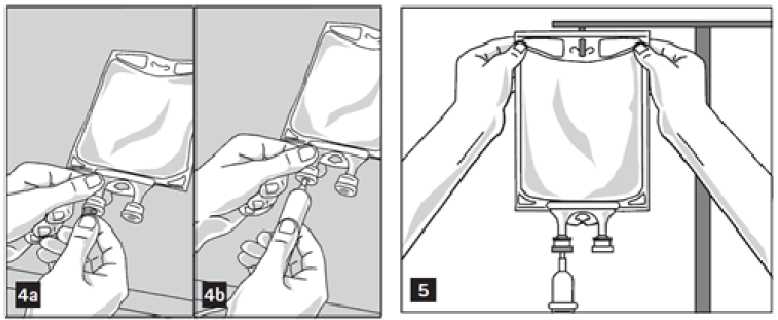
1. Remove aluminium foil of infusion port (green colour) at the bottom of container (figure 4a) and attach administration set (figure 4b): use non-vented infusion set or close air vent on a vented set. Follow directions for use of the infusion set. Please note: The area underneath the foil of the infusion port is sterile.
2. Hang bag on an i.v. pole (figure 5).
Additional Information:
Container is PVC-free, DEHP-free, latex-free.
7 MARKETING AUTHORISATION HOLDER
B.Braun Melsungen AG Carl-Braun-StraBe 1
34212 Melsungen Germany
Postal address:
34209 Melsungen Germany
Phone: +49/5661/71-0 Fax: +49/5661/71-4567
8 MARKETING AUTHORISATION NUMBER(S)
PL 03551/0133
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
20/06/2013
10 DATE OF REVISION OF THE TEXT
28/06/2013