Artelac 0.32%W/V Eye Drops Single Dose Unit
|
Graph ics& Packing mm mm _ _ _ _ km mm Technology Section ULOTKA PIL RAUSCH 1 LOMR ICN POLFA RZESZOW S.A. ™ | |||||
|
Nazwa produktu Product Name |
Artelac 0.32% w/v DRP |
Kolor nadruku Colours | |||
|
Black | |||||
|
Kraj Country (ISO) |
United Kingdom (UK) |
Nr wykrojnika Spec No |
930937 | ||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
40898PB276/6-UK | |||
|
Nr korekty Proof No |
6 30.07.2015 Date |
Kod farmaceutyczny Pharmacode |
329 | ||
|
Kod wersji Valeant version code |
P9UKIE01 |
Inny kod Other code |
- | ||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
148 x 210 mm | ||
|
Kroj czcionki Font used |
Helvaticafont Family |
Gramatura papieru Paper weight |
- g/m2 | ||
|
Komentarze Comments (Reason for the change) | |||||
|
safety update to include phosphate warning and qrd update PMRJCN-15-0625-A Packing site: DMP Berlin | |||||
|
Akceptacja Techniczna Technical Approval | |||||
|
GENEHMIGT Von Carsten Lorenz, 15:01, 30.07.2015 |
' i / | ||||
|
Akceptacja Dziafu ds. Rejestracji Regulatory Affairs Dept. Approval | |||||
PACKAGE LEAFLET: INFORMATION FOR THE USER

0.32% w/v eye drops SINGLE DOSE UNIT PRESERVATIVE FREE
The name of your medicine is Artelac 0.32% w/v eye drops Single Dose Unit Preservative Free and it is called Artelac SDU Preservative Free in this leaflet.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- You must talk to a doctor if you do not feel better or if you feel worse after number of days.
What is in this leaflet
1. What Artelac SDU Preservative Free is and what it is used for
2. What you need to know before you take Artelac SDU Preservative Free
3. How to take Artelac SDU Preservative Free
4. Possible side effects
5. How to store Artelac SDU Preservative Free
6. Contents of the pack and other information
1. WHAT ARTELAC SDU PRESERVATIVE FREE IS AND WHAT IT IS USED FOR
Artelac SDU Preservative Free contains hypromellose as the active ingredient, which is a form of cellulose.
Your drops are used as a tear substitute and lubricant when applied to the eye.
The hypromellose in these drops increases the thickness of the solution which has a moisturising effect and helps the solution to stay in contact with the eye for a longer period of time.
These drops work by soothing the eye and giving tear-like lubrication to the eyes and eye lids.
They are used to treat the symptoms of "dry eye", which is a dehydration of the surface of the eye because no natural tears are being produced. Dry eye can also be caused if it is not possible to close your eyelids partly or completely. You must talk to a doctor if you do not feel better or if you feel worse.
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE ARTELAC SDU PRESERVATIVE FREE
Do not use Artelac SDU Preservative Free
■ if you are allergic to hypromellose or any of the ingredients of this medicine (listed in section 6).
Warnings and precautions
■ Talk to your doctor or pharmacist before taking Artelac SDU Preservative Free
■ if you wear soft contact lenses - take out your lenses before using Artelac SDU Preservative Free and then wait for at least 15 minutes after using these drops before putting your lenses back in.
Other medicines and Artelac SDU Preservative Free
■ Tell your doctor or pharmacist if you are taking , have recently taken or might take any other medicines.
■ You should wait 5 minutes before using any other eye medication.
Pregnancy, breast-feeding and fertility
■ If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
■ All eye drops can cause a short term blurring of vision when first used so you should wait until your eyes are clear before driving or using machines.
3. HOW TO TAKE ARTELAC SDU PRESERVATIVE FREE
Always use your drops exactly as your doctor or pharmacist has told you to. Check with your doctor or pharmacist if you are not sure.
The medicine is to be used only as drops applied into one or both eye(s).
The recommended dose is
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
The usual dose is to apply one drop in the corner of the eye, nearest the nose, 3 to 5 times each day or as required to give enough lubrication.
Dry eye is a condition that varies from person to person. Use the drops as often as you think necessary.
continued overleaf
P9UKIE01_40898PB276-6-UK_Artelac_032_w-v_DR.P_30_60_120_SDU_UK_IE_vl.indd 1
329 6Z£
2015-07-30 14:51:30
|
Graphics&Packing m u u ** ** u u u ■ ^ m m w% Technology Section ULOTKA PIL RAUSCH 1 LOMB ICN POLFA RZESZOW S.A. w ■ ■ ■ “ | |||||
|
Nazwa produktu Product Name |
Artelac 0.32% w/v DRP I ~ |
Kolor nadruku Colours | |||
|
mm | |||||
|
Black | |||||
|
Kraj Country (ISO) |
United Kingdom (UK) |
Nr wykrojnika Spec No |
930937 | ||
|
Opracowane przez Designed by |
Kod Wytworcy Manufacturer code |
40898PB276/6-UK | |||
|
Nr korekty Proof No |
6 °a[a 30.07.2015 Date |
Kod farmaceutyczny Pharmacode |
329 | ||
|
Kod wersji Valeant version code |
P9UKIE01 |
Inny kod Other code |
- | ||
|
Rozmiar czcionki Font size |
minimum 8 pt |
Wymiar ulotki PIL size |
148x210 mm | ||
|
Kroj czcionki Font used |
Helvatica font Family |
Gramatura papieru Paper weight |
- g/m2 | ||
|
Komentarze Comments (Reason for the change) | |||||
|
safety update to include phosphate warning and qrd update PMRJCN-15-0625-A Packing site: DMP Berlin | |||||
|
Akceptacja Techniczna Technical Approval | |||||
|
[' | |||||
|
Akceptacja Dzialu ds. Rejestracji Regulatory Affairs Dept. Approval | |||||
Directions for use
- sit down in front of a mirror so that you can see what you are doing
- wipe your eyes to clear any residual wateriness or discharge
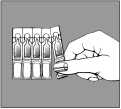
- holding the unit by the label end, remove a single-dose unit from the pack
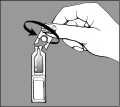
- twist the cap off the single-dose unit
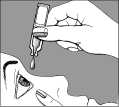
- pull your lower eyelid gently down, and then carefully place one drop inside the lower eyelid, in the corner nearest the nose
- release the lower eyelid, and blink a few times to make sure the eye is covered by the liquid
- repeat the procedure for your other eye
- when you have finished, throw away the single-dose unit Always read the label before using this medicine.
If you use more Artelac SDU Preservative Free than you should
If you accidentally use a larger dose than recommended, this may cause some blurring of vision,
which will soon pass.
Make sure you know how and when to use your medicine. If you are not sure about using your drops, ask your pharmacist.
If you forget to take Artelac SDU Preservative Free Do not take a double dose to make up for a forgotten dose 4. POSSIBLE SIDE EFFECTS
Like all medicines, Artelac SDU Preservative Free can cause some side effects, although not everybody gets them. If you experience any difficulty stop using your drops and talk to your doctor or pharmacist.
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Like all eye drops you may briefly have blurred vision or a slight stinging feeling after using your drops.
There is also a slight possibility that irritation or itching of the eye may occur in a very small number of people.
(frequency cannot be estimated from the available data)
If any side effect gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ARTELAC SDU PRESERVATIVE FREE
■ Keep out of the reach and sight of children.
■ Do not use this medicine after its expiry date which is stated on the label.
The expiry date refers to the last day of that month.
If the expiry date has passed, take the medicine back to your pharmacist.
■ Keep the eye drops at normal room temperature (do not store above 25°C).
■ Protect from light.
■ Your eye drops are sterile until first opened. It is important to keep the drops as clean as possible during use.
■ Your drops come in single-dose, single-use units, which should be thrown away immediately after use.
■ Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Artelac SDU Preservative Free contains
The active ingredient is Hypromellose 0.32% w/v.
The other ingredients are Sorbitol, Disodium Phosphate Dodecahydrate, Sodium Dihydrogen Phosphate Dihydrate and Purified Water.
What Artelac SDU Preservative Free looks like and contents of the pack
Your eye drops are artificial tears and are supplied in plastic single-dose units in pack sizes of 30, 60 and 120.
Each single dose unit has a volume of 0.5 ml. Your drops contain no preservative, therefore each unit is for single-use only. These eye drops are a clear solution.
Your drops do not contain a preservative and can be used by patients who are hypersensitive to preservatives or who wear contact lenses.
This medicine is for you, never give it to anyone else, even if they appear to have the same symptoms as you.
Return any unused medicine to your pharmacist.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation Holder is: Bausch & Lomb UK Ltd.,
106 London Road, Kingston-Upon-Thames, Surrey,
PL 03468/0033
This leaflet was last approved in 07/2015.
The manufacturer is:
Dr Gerhard Mann Chem-pharm Fabrik GmbH 13581 Berlin
Germany P9UKIE01
40898PB276/6-UK
P9UKIE01 40898PB276-6-UK Artelac 032 w-v DRP 30 60 120 SDU UK IE vl.indd 2
2015-07-30 14:51:30