Artelac 0.32%W/V Eye Drops Single Dose Unit
Artelac® 0.32% w/v Eye Drops Single Dose Unit
_(hypromellose)_
PATIENT INFORMATION LEAFLET
The name of your medicine is Artelac 0.32% w/v Eye Drops Single Dose Unit but it will be referred to as Artelac throughout this leaflet.
Please read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription, as an over the counter item only in a pharmacy. However, you still need to use Artelac carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve.
• If any of the side effects gets serious, or if you notice any side effect not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Artelac is and what it is used for
2. Before you use Artelac
3. How to use Artelac
4. Possible side effects
5. How to store Artelac
6. Further information
1. WHAT ARTELAC IS AND WHAT IT IS USED FOR
Your drops contain hypromellose as the active ingredient, which is a form of cellulose.
Your drops are used as a tear substitute and lubricant when applied to the eye.
The hypromellose in these drops increases the thickness of the solution which has a moisturising effect and helps the solution to stay in contact with the eye for a longer period of time.
These drops work by soothing the eye and giving tear-like lubrication to the eyes and eyelids.
They are used to treat the symptoms of "dry eye", which is a dehydration of the surface of the eye because no natural tears are being produced. Dry eye can also be caused if it is not possible to close your eyelids partly or completely.
2. BEFORE YOU USE ARTELAC
Do not use these drops
• if you have been told, or know, that you are allergic (hypersensitive) to any of its ingredients (see section 6).
Take special care with these drops
• if you wear soft contact lenses - take out your lenses before using Artelac and then wait for at least 15 minutes after using these drops before putting your lenses back in.
Using other medicines
• You should wait 5 minutes before using any other eye medication.
Pregnancy and breast-feeding
• These drops can be used if you are pregnant or breast-feeding.
Driving and using machines
• All eye drops can cause a short term blurring of vision when first used so you should wait until your eyes are clear before driving or using machines.
Page
3. HOW TO USE ARTELAC
Always use your drops exactly as your doctor or pharmacist has told you to. You should check with your doctor or pharmacist if you are not sure.
The medicine is to be used only as drops applied into one or both eye(s).
Dosage
The usual dose is to apply one drop in the corner of the eye, nearest the nose, 3 to 5 times each day or as required to give enough lubrication.
Dry eye is a condition that varies from person to person. Use the drops as often as you think necessary.
Directions for use
• sit down in front of a mirror so that you can see what you are doing
• wipe your eyes to clear any residual wateriness or discharge
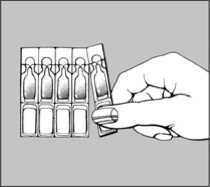
• holding the unit by the label end, remove a single dose unit from the pack
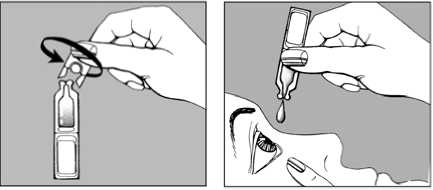
• twist the cap off the single dose unit
• pull your lower eyelid gently down, and then carefully place one drop inside the lower eyelid, in the corner nearest the nose
• release the lower eyelid, and blink a few times to make sure the eye is covered by the liquid
• repeat the procedure for your other eye
• when you have finished, throw away the single dose unit Always read the label before using this medicine.
If you use more Artelac than you should
If you accidentally use a larger dose than recommended, this may cause some blurring of vision, which will soon pass.
Make sure you know how and when to use your medicine. If you are not sure about using your drops, ask your pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Artelac can cause some side effects, although not everybody gets them.
If you experience any difficulty stop using your drops and talk to your doctor or pharmacist.
Like all eye drops you may briefly have blurred vision or a slight stinging feeling after using your drops.
There is also a slight possibility that irritation or itching of the eye may occur in a very small number of people.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE ARTELAC
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not use this medicine after its expiry date.
Check the expiry date on the label before you begin to use your medicine.
The expiry date refers to the last day of that month.
If the expiry date has passed, take the medicine back to your pharmacist.
• Do not store above 25°C.
• Your eye drops are sterile until first opened. It is important to keep the drops as clean as possible during use.
• Your drops come in single dose, single-use units, which should be thrown away immediately after use.
• If your medicine appears to be discoloured or show any other signs of deterioration, please return to your pharmacist who will advise you further.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Artelac contains
The active ingredient is hypromellose 0.32% w/v. Each ml contains 3.2mg hypromellose.
Also contains sorbitol, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate and water for injections.
What Artelac looks like and contents of the pack
Artelac is a sterile, clear solution supplied in 0.5ml plastic single dose units.
Artelac is available in packs containing 30 and 60 single dose units.
Artelac does not contain a preservative and can be used by patients who are hypersensitive to preservatives or who wear contact lenses.
Manufacturer
Manufactured by: Laboratoire CHAUVIN, Z.I. Ripotier Haut, 07200 Aubenas, France.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Ltd., Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: Landmark Pharma Ltd., 7 Regents Drive, Prudhoe, Northumberland, NE42 6PX. PL No: 21828/0602 [T]
Leaflet issue and revision date (Ref.): 09.05.14
Artelac® is a registered trademark of Dr. Gerhard Mann, Chem-Pharm. Fabrik GmbH.
Page 2 of 2