Artiss Solutions For Sealant Deep Frozen
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
ARTISS
Solutions for Sealant, deep frozen
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Component 1:
Sealer Protein Solution Human Fibrinogen (as clottable protein)
Aprotinin (synthetic)
Component 2:
Thrombin Solution Human Thrombin 4 IU3/ml
Calcium Chloride 40 ^mol/ml
1 prefilled double chamber syringe which contains Sealer Protein Solution (with Aprotinin), deep frozen <1 ml><2 ml><5 ml>, in one chamber and Thrombin Solution (with Calcium Chloride), deep frozen<1 ml><2 ml><5 ml>, in the other chamber results in <2 ml><4 ml><10 ml> total volume of product ready for use.
|
After mixing |
1 ml |
2 ml |
4 ml |
10 ml |
|
Component 1: Sealer protein solution Human Fibrinogen |
45.5 mg |
91 mg |
182 mg |
455 mg |
|
(as clottable protein) Aprotinin (synthetic) |
1,500 KIU |
3,000 KIU |
6,000 KIU |
15,000 KIU |
|
Component 2: Thrombin Solution Human Thrombin |
2 IU |
4 IU |
8 IU |
20 IU |
|
Calcium Chloride |
20 pmol |
40 pmol |
80 pmol |
200 pmol |
ARTISS contains Human Factor XIII co-purified with Human Fibrinogen in a range of 0.6 - 5 IU/ml.
1
2
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solutions for Sealant Deep frozen
Colourless to pale yellow and clear to slightly turbid solutions. Component 1, Sealer Protein Solution: pH 6.5 - 8.0
Component 2, Thrombin Solution: pH 6.0 - 8.0
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
ARTISS is indicated as a tissue glue to adhere/seal subcutaneous tissue in plastic, reconstructive and burn surgery, as a replacement or an adjunct to sutures or staples (see 5.1). In addition, ARTISS is indicated as an adjunct to hemostasis on subcutaneous tissue surfaces.
4.2 Posology and method of administration
ARTISS is intended for hospital use only. The use of ARTISS is restricted to experienced surgeons who have been trained in the use of ARTISS.
Posology
The amount of ARTISS to be applied and the frequency of application should always be oriented towards the underlying clinical needs of the patient.
The dose to be applied is governed by variables including, but not limited to, the type of surgical intervention, the size of the area and the mode of intended application, and the number of applications.
Application of the product must be individualized by the treating physician. In clinical trials, the individual dosages have typically ranged from 0.2-12 ml. For some procedures (e.g. the sealing of large burned surfaces), larger volumes may be required.
The initial amount of the product to be applied at a chosen anatomic site or target surface area should be sufficient to entirely cover the intended application area. The application can be repeated, if necessary, to any small areas that may have not been previously treated. However, avoid reapplication of ARTISS to a pre-existing polymerized ARTISS layer as ARTISS will not adhere to a polymerized layer.
It is recommended that the initial application covers the entire intended application area.
As a guideline for the gluing of surfaces, 1 pack of ARTISS 2 ml (i.e., 1 ml Sealer Protein Solution plus 1 ml Thrombin Solution) will be sufficient for an area of at least 10 cm2.
The skin graft should be attached to the wound bed immediately after ARTISS has been applied. The surgeon has up to 60 seconds to manipulate and position the graft prior to polymerization. After the flap or graft has been positioned, hold in the desired position by gentle compression for at least 3 minutes to ensure ARTISS sets properly and the graft or flap adheres firmly to the underlying tissue.
The required amount of ARTISS depends on the size of the surface to be covered. The approximate surface areas covered by each pack size of ARTISS by spray application are:
|
Approximate area requiring tissue |
Required pack size of ARTISS |
|
adherence | |
|
100 cm2 |
2 ml |
|
200 cm2 |
4 ml |
|
500 cm2 |
10 ml |
To avoid the formation of excess granulation tissue and to ensure gradual absorption of the solidified fibrin sealant, only a thin layer of the mixed Sealer Protein -Thrombin Solution should be applied.
ARTISS has not been administered to patients > 65 years old in clinical trials. Paediatric Population
Currently available data are described in section 5.1 but no recommendation on a posology can be made.
Method of administration
For epilesional (topical) use. Do not inject.
For subcutaneous use only. ARTISS is not recommended for laparoscopic surgery.
In order to ensure optimal safe use of ARTISS it should be sprayed using a pressure regulator device that delivers a maximum pressure of up to 2.0 bar (28.5 psi).
Prior to applying ARTISS the surface area of the wound needs to be dried by standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas for drying the site.
ARTISS must be sprayed only onto application sites that are visible.
ARTISS should only be reconstituted and administered according to the instructions and with the devices recommended for this product (see section 6.6).
For spray application, see sections 4.4 and 6.6 for specific recommendations on the required pressure and distance from tissue per surgical procedure and length of applicator tips.
4.3 Contraindications
ARTISS is not indicated to replace skin sutures intended to close surgical wounds. ARTISS alone is not indicated for the treatment of massive and brisk arterial or venous bleeding.
ARTISS must never be applied intravascularly.
ARTISS is contraindicated in the case of hypersensitivity to the active substances or to any of the excipients (see also section 4.4).
4.4 Special warnings and precautions for use
For epilesional use only. Do not apply intravascularly. Life threatening
thromboembolic complications may occur if the preparation is applied intravascularly.
Soft tissue injection of ARTISS carries the risk of local tissue damage.
Caution must be used when applying fibrin sealant using pressurized air or gas.
• Any application of pressurized air or gas is associated with a potential risk of air or gas embolism, tissue rupture, or gas entrapment with compression, which may be life-threatening or fatal.
• Apply ARTISS as a thin layer. Excessive clot thickness may negatively interfere with the product's efficacy and the wound healing process.
• Life-threatening/fatal air or gas embolism has occurred with the use of spray devices employing a pressure regulator to administer fibrin sealants. This event appears to be related to the use of the spray device at higher than recommended pressures and/or in close proximity to the tissue surface. The risk appears to be higher when fibrin sealants are sprayed with air, as compared to CO2 and therefore cannot be excluded with ARTISS when sprayed in open wound surgery.
• When applying ARTISS using a spray device, be sure to use a pressure within the pressure range recommended by the spray device manufacturer (see table in section 6.6 for pressures and distances).
• ARTISS spray application should only be used if it is possible to accurately judge the spray distance as recommended by the manufacturer. Do not spray closer than the recommended distances.
• When spraying ARTISS, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism (also see section 4.2).
• ARTISS must not be used with the Easy Spray / Spray Set system in enclosed body areas.
• Only use application devices CE marked for the administration of ARTISS.
ARTISS is not indicated for hemostasis and sealing in situations where a fast clotting of the sealant is required. Especially in cardiovascular procedures in which sealing of vascular anastomoses is intended ARTISS should not be used.
ARTISS is not indicated for use in neurosurgery and as a suture support for gastrointestinal anastomoses or vascular anastomoses as no data are available to support these indications.
Before administration of ARTISS care is to be taken that parts of the body outside the designated application area are sufficiently protected/covered to prevent tissue adhesion at undesired sites.
Oxycellulose-containing preparations may reduce the efficacy of ARTISS and should not be used as carrier materials (see Section 6.2).
As with any protein-containing product, allergic type hypersensitivity reactions are possible. Signs of hypersensitivity reactions may include hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If these symptoms occur, the administration must be discontinued immediately.
ARTISS contains aprotinin. Even in case of strict local application, there is a risk of anaphylactic reaction linked to the presence of aprotinin. The risk seems to be higher in cases where there was previous exposure, even if it was well tolerated. Therefore any use of aprotinin or aprotinin containing products should be recorded in the patients’ records.
As synthetic aprotinin is structurally identical to bovine aprotinin the use of ARTISS in patients with allergies to bovine proteins should be carefully evaluated.
In the event of anaphylactic/anaphylactoid or severe hypersensitivity reactions, administration is to be discontinued. If possible, remove any applied, polymerized product from the surgical site. Adequate medical treatment and provisions should be available for immediate use in the event of an anaphylactic reaction. State-of-the-art emergency measures are to be taken. In case of shock, standard medical treatment for shock should be implemented.
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses or other pathogens.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV).
The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g., hemolytic anemia).
It is strongly recommended that every time that ARTISS is administered to the patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
4.5 Interaction with other medicinal products and other forms of interaction
No formal interaction studies have been performed.
Similar to comparable products or thrombin solutions, the product is denatured after exposure to solutions containing alcohol, iodine or heavy metals (e.g. antiseptic solutions). Such substances should be removed to the greatest possible extent before applying the product.
See section 4.4 or 6.2 for substances that can interfere with the product’s performance.
4.6 Fertility, pregnancy and lactation
The safety of fibrin sealants/haemostatics for use in human pregnancy or breastfeeding has not been established in controlled clinical trials. Animal studies have also not been performed.
Therefore, the product should be administered to pregnant and lactating women only if clearly needed.
See section 4.4 for information on Parvovirus B19 infection.
The effects of ARTISS on fertility have not been established.
4.7 Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
Intravascular injection could lead to thromboembolic events and disseminated intravascular coagulation (DIC) and there is also a risk of anaphylactic reactions (see section 4.4).
Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the application site, bradycardia, bronchospasm, chills, dyspnoea, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, pruritus, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) may occur in rare cases in patients treated with fibrin sealants/hemostatics.
In isolated cases, these reactions have progressed to severe anaphylaxis. Such reactions may especially be seen if the preparation is applied repeatedly, or administered to patients known to be hypersensitive to aprotinin (see section 4.4) or any other constituents of the product.
Even if a first treatment with ARTISS was well tolerated, a subsequent administration of ARTISS or systemic administration of aprotinin may result in severe anaphylactic reactions.
Antibodies against components of fibrin sealant may rarely occur.
For safety with respect to transmissible agents, see section 4.4.
Life threatening/fatal air or gas embolism when using devices with pressurized air or gas occurred; this event appears to be related to an inappropriate use of the spray device (e.g. at higher than recommended pressures and in close proximity of the tissue surface).
Adverse reactions summarized in the table below were reported from clinical studies of ARTISS and from post-marketing experience with Baxter Fibrin Sealants (marked with a p in the adverse event table). Known frequencies of these adverse reactions are based on a controlled clinical study in 138 patients where skin grafts were fixed to excised burn wounds using ARTISS. None of the events observed in the clinical study were classified as serious.
The ADRs and their frequencies are summarized below:
Common (>1/100 to <1/10)
Uncommon (>1/1000 to <1/100)
Not known (cannot be estimated from the available data)
|
Table 1 | ||
|
Adverse Reactions | ||
|
System organ class (SOC) |
Preferred MedDRA Term |
Frequency |
|
Skin and subcutaneous tissue disorders |
Dermal cyst |
uncommon |
|
Pruritus |
common | |
|
Injury, poisoning and procedural complications |
Skin graft failure |
common |
|
Vascular disorders |
Air embolismp due to an inappropriate use of the spray device (see section 4.4) |
not known |
p Adverse events observed in post-marketing experience with Baxter Fibrin Sealants.
Class Reactions
Other adverse reactions associated with products of the fibrin sealant/hemostatic class include: Hypersensitivity reactions which could manifest as application site irritation, chest discomfort, chills, headache, lethargy, restlessness and vomiting. Further class reactions are: Anaphylactic reaction, bradycardia, tachycardia, hypotension,
haematoma, dyspnoea, nausea, urticaria, flushing, impaired healing, oedema, pyrexia and seroma.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No case of overdose has been reported
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: local hemostatics, ATC code: B02BC; tissue adhesives, ATC code: V03A K
ARTISS can replace sutures or staples when used for fixation of skin grafts to burned or otherwise injured wound areas. ARTISS can be used as an adjunct to sutures or staples to adhere and seal skin flaps in cases where sutures/staples are expected to yield unsatisfactory results with respect to postoperative hematoma or seroma formation.
The fibrin adhesion system initiates the last phase of physiological blood coagulation. Conversion of fibrinogen into fibrin occurs by the splitting of fibrinogen into fibrin monomers and fibrinopeptides. The fibrin monomers aggregate and form a fibrin clot. Factor XIIIa, which is activated from factor XIII by thrombin, crosslinks fibrin. Calcium ions are required for the conversion of fibrinogen and the crosslinkage of fibrin.
As wound healing progresses, increased fibrinolytic activity is induced by plasmin, and decomposition of fibrin to fibrin degradation products is initiated. Proteolytic degradation of fibrin is inhibited by anti-fibrinolytics. Aprotinin is present in ARTISS (frozen) as an antifibrinolytic to prevent premature degradation of the clot.
For efficacy, in vivo studies in an animal model closely imitating the situation in patients were used. ARTISS (frozen and lyophilized presentations) demonstrated efficacy regarding sealing autologous split skin grafts and mesh grafts.
ARTISS (frozen) was investigated for fixation of split thickness sheet skin grafts in burn patients in a prospective, randomised, controlled, multicenter clinical study. In each of the 138 patients, two comparable test sites were identified. In one test site the skin graft was fixed with ARTISS in the other test site the graft was fixed with staples (control). ARTISS proved to be non-inferior to staples with respect to the primary efficacy endpoint, complete wound closure at Day 28 was evaluated by a blinded evaluator panel from photographs. This was achieved in 55/127 patients (43.3%) treated with ARTISS (frozen) and 47/127 patients (37%) treated with staples.
With respect to secondary endpoints, ARTISS showed a significantly lower incidence and size of hematoma/seroma on Day 1 (p < 0.0001 for incidence as well as size). Incidence and area of engraftment on Day 5 and wound closure on Day 14, as well as area of wound closure on Day 28 were not different. ARTISS was also superior to staples with respect to patient satisfaction (p < 0.0001) and patients experienced significantly less anxiety about pain with ARTISS than with staples (p < 0.0001). Moreover, ARTISS was significantly superior to staples with respect to the investigator's assessment of quality of graft adherence, preference of fixation method and satisfaction with graft fixation, overall quality of healing and overall rate of healing (p < 0.0001).
Thirty-seven (37) pediatric patients aged 1.1 to 18 years were evaluated in this trial. Eighteen (18) of these patients were 6 years old or younger.
Dosage used in clinical trials was the same for pediatric and adult patients.
5.2 Pharmacokinetic properties
ARTISS is intended for epilesional use only. Intravascular administration is contraindicated. As a consequence, intravascular pharmacokinetic studies were not performed in man.
Pharmacokinetic studies in different species of laboratory animals were not conducted.
Fibrin sealants/hemostatics are metabolized in the same way as endogenous fibrin by fibrinolysis and phagocytosis.
5.3 Preclinical safety data
No preclinical safety data are available for ARTISS (thrombin 4 IU/ml). Toxicity studies were done with Fibrin Sealants containing thrombin 500 IU/ml, as representative for products containing thrombin 4 IU/ml. Single-dose toxicity studies in rats and rabbits indicated no acute toxicity of Fibrin Sealant VH S/D (500 IU/ml). Fibrin Sealant VH S/D (500 IU/ml) also proved well tolerated in wound healing models in rats and rabbits, and in in vitro human fibroblast cultures.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Component 1: Sealer Protein Solution Human Albumin Solution L-Histidine Niacinamide
Polysorbate 80 (Tween 80)
Sodium Citrate Dihydrate Water for Injections
Component 2: Thrombin Solution Human Albumin Solution Sodium Chloride Water for Injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. Oxycellulose-containing preparations may reduce the efficacy of ARTISS and should not be used as carrier materials.
6.3 Shelf life
12 months
6.4. Special precautions for storage
Store and transport frozen (at <-20°C).
Keep the syringe in the outer carton in order to protect from light.
Unopened pouches, thawed at room temperature, may be stored for up to 14 days at controlled room temperature (not exceeding +25°C). Do not refreeze or refrigerate after thawing.
6.5 Nature and contents of container
1 ml, 2 ml, or 5 ml of sealer protein solution and 1, 2 or 5 ml of Thrombin Solution in a single-use double-chamber syringe (polypropylene) with a tip-cap in a bag , and one device set with one double syringe plunger, 2 joining pieces and 4 application cannulae.
Pack size of 1 (1 x 1 ml + 1 ml, 1 x 2 ml + 2 ml, 1 x 5 ml + 5 ml)
Both Sealer Protein Solution and Thrombin Solution are contained in a single-use double-chamber syringe made of polypropylene.
Not all pack sizes may be marketed.
Other accessories for application of the product can be obtained from BAXTER.
6.6 Special precautions for disposal
General
To prevent ARTISS from adhering to gloves and instruments, wet these with sodium chloride solution before contact.
As a guideline for the gluing of surfaces, 1 pack of ARTISS 2 ml (i.e., 1 ml Sealer Protein Solution plus 1 ml Thrombin Solution) will be sufficient for an area of at least 10 cm2.
The required dose of ARTISS depends on the size of the surface to be covered. Handling and Preparation
The inner bag and its contents are sterile unless the integrity of the outside package is compromised.
It is recommended to thaw and warm the two sealant components using a sterile water bath at a temperature of 33 - 37°C. The water bath must not exceed a temperature of 37°C. (In order to control the specified temperature range, the water temperature should be monitored using a thermometer and the water should be changed as necessary. When using a sterile water bath for thawing and warming, the pre-filled double chamber syringe assembly should be removed from the aluminum-coated plastic bags.)
The protective syringe cap should not be removed until thawing is complete and the joining piece is ready to be attached. Do not use ARTISS unless it is completely thawed and warmed (liquid consistency).
Thaw pre-filled syringes using one of the following options:
1. Room Temperature Thawing (not exceeding +25°C):
The product can be thawed at room temperature. Times given in Table 1 are minimum times for thawing at room temperature. The maximum time the product can be kept (in both aluminum-coated plastic bags) at room temperature is 14 days.
When thawing at room temperature, the product must be additionally warmed to 33°C - 37°C in an incubator just before use. Respective warming times in the incubator are also given in Table 1.
Table 1: Thawing times at Room Temperature (= RT) followed by additional warming, prior to use, in an Incubator at 33°C to a maximum of 37°C
|
Pack Size |
Thawing Times at Room Temperature (Product in aluminum-coated plastic bags) |
Warming Times at 33-37°C in Incubator after Thawing at RT (Product in aluminum-coated plastic bags) | ||
|
2 ml |
60 minutes |
+ |
15 minutes | |
|
4 ml |
110 minutes |
+ |
25 minutes | |
|
10 ml |
160 minutes |
+ |
35 minutes | |
Once ARTISS has been warmed up to 33 - 37°C the product may be stored for up to 4 hours.
2. Quick Thawing:
Table 2: Thawing and Warming Times with Sterile Water Bath at 33°C to a maximum of 37°C
|
Pack Size |
Thawing and Warming Times (Product removed from aluminum-coated plastic bags) |
|
2 ml |
5 minutes |
|
4 ml |
5 minutes |
|
10 ml |
12 minutes |
Transfer plunger and the inner pouch to the sterile field, remove prefilled syringe from inner pouch and place directly into sterile water bath. Ensure the contents of the prefilled syringe are completely immersed in water._
A third alternative is to thaw the product off the sterile field using a non-sterile water bath.
Maintain the prefilled syringe in both pouches and place into a water bath off the sterile field for an appropriate time (see Table 3). Ensure the pouches remain submerged throughout thawing. Remove from the water bath after thawing, dry external pouch and transfer inner pouch with prefilled syringe and plunger to the sterile field.
Table 3: Thawing and Warming times off the Sterile Field with Non-Sterile Water Bath at 33°C to a maximum of 37°C
|
Pack Size |
Thawing and Warming Times (Product in aluminum-coated plastic bags) |
|
2 ml |
30 minutes |
|
4 ml |
40 minutes |
|
10 ml |
80 minutes |
Alternatively, the sealant components may be thawed and warmed in an incubator between 33°C and 37°C. The thawing and warming times in the incubator are indicated in Table 4 below. The times refer to product in the aluminum-coated plastic bags.
Table 4: Thawing and Warming Times in Incubator at 33°C to a maximum of 37°C
|
Pack Size |
Thawing and Warming Times in Incubator (Product in aluminum-coated plastic bags) |
|
2 ml |
40 minutes |
|
4 ml |
85 minutes |
|
10 ml |
105 minutes |
Note: Do not thaw by holding product in your hands.
Do not microwave.
After thawing do not refrigerate or refreeze.
After Quick Thawing (i.e. thawing at a temperature of 33 - 37°C) ARTISS may be stored at 33 - 37°C for a maximum of 4 hours.
To facilitate optimal blending of the two solutions, the two sealant components must be warmed to 33 - 37°C immediately before use. (The temperature of 37°C must, however, not be exceeded!)
The Sealer Protein and the Thrombin Solutions should be clear or slightly opalescent. Do not use solutions that are cloudy or have deposits. Thawed products should be inspected visually for particulate matter and discoloration prior to administration or any variation in physical appearance. In the event of either being observed, discard the solution.
The thawed Sealer Protein Solution should be a slightly viscous liquid. If the solution has the consistency of a solidified gel, it must be assumed to have become denatured (e.g., due to an interruption of the cold storage chain or by overheating during warming). In this case, ARTISS must not be used. Unopened pouches, thawed at room temperature, may be stored for up to 14 days at controlled room temperature (not exceeding +25°C). If not used within 14 days after thawing, ARTISS has to be discarded.
The protective syringe cap should not be removed until thawing is complete and the joining piece is ready to be attached. Do not use ARTISS unless it is completely thawed and warmed (liquid consistency).
For further preparation instructions please refer to the responsible nurse or medical doctor.
ADMINISTRATION
For application, the double-chamber syringe with the Sealer Protein Solution and the Thrombin Solution has to be connected to a joining piece and an application cannula as provided in the accompanying set of devices. The common plunger of the double-chamber syringe ensures that equal volumes are fed through the joining piece before being mixed in the application cannula and ejected.
Operating Instructions
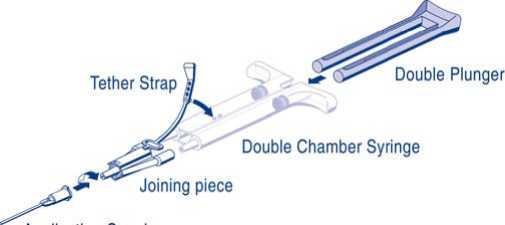
Application Canula
- Connect the nozzles of the double-chamber syringe to the joining piece ensuring that they are firmly fixed. Secure the joining piece by fastening the tether strap to the double-chamber syringe. If the tether strap tears, use the spare joining piece. If none is available, further use is still possible but tightness of the connection needs to be ensured to prevent any risk of leaking.
- Fit an application cannula onto the joining piece.
- Do not expel the air remaining inside the joining piece or application cannula until you start actual application as the aperture of the cannula may clog otherwise.
- Immediately before application expel and discard the first several drops from the application cannula to ensure adequately mixed product
- Apply the mixed Sealer Protein - Thrombin Solution onto the recipient surface or surfaces of the parts to be sealed.
If application of the fibrin sealant components is interrupted, clogging may occur in the cannula. Replace the application cannula with a new one only immediately before application is resumed. If the apertures of the joining piece are clogged, use the spare joining piece provided in the package.
Application is also possible with other accessories supplied by BAXTER that are particularly suited for, e.g. minimally invasive surgery, application to large or difficult-to-access areas. When using these application devices, strictly follow the Instructions for Use of the devices.
Spray application
When applying ARTISS using a spray device be sure to use a pressure and a distance from tissue within the ranges recommended by the manufacturer as follows:
|
Spray set to be used |
Applicator tips to be used |
Pressure regulator to be used |
Recommended distance from target tissue |
Recommended spray pressure | |
|
Open wound surgery of subcutane ous tissue |
Tisseel / Artiss Spray Set |
n.a. |
EasySpray |
10 - 15 cm |
1.5-2.0 bar (21.5-28.5 psi) |
|
Tisseel / Artiss Spray Set 10 pack |
n.a. |
EasySpray |
When spraying the ARTISS, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism (see sections 4.2 and 4.4).
Disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Baxter Healthcare Caxton Way Thetford Norfolk IP24 3SE United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 00116/0634
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
11/03/2009
10 DATE OF REVISION OF THE TEXT
06/11/2015
Contained in a total protein concentration of 96 - 125 mg/ml
EPU (European Pharmacopoeia Unit) corresponds to 1800 KIU (Kallidinogenase Inactivator Unit)
Thrombin activity is calculated using the current WHO International Standard for Thrombin.