Bador 20 Mg/Ml Eye Drops Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Bador 20 mg/ml Eye Drops, Solution
dorzolamide-hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Bador is and what it is used for
2. Before you use Bador
3. How to use Bador
4. Possible side effects
5. How to store Bador
6. Further information
1. WHAT BADOR IS AND WHAT IT IS USED FOR
Bador contains dorzolamide which belongs to a group of medicines known as “carbonic anhydrase inhibitors”, and reduces pressure within the eye.
Bador is prescribed to lower raised pressure within the eye in the treatment of glaucoma, when beta-blocker eye drops alone are not adequate and in patients who must not use beta-blockers.
2. BEFORE YOU USE BADOR
Do not use Bador
• if you are allergic (hypersensitive) to dorzolamide hydrochloride or any of the other ingredients of Bador
• if you suffer from severe kidney disease or kidney problems, or if you have ever had kidney stones
• if you suffer from a condition where the blood turns acidic, due to high chloride levels (hyperchloraemic acidosis).
If you are not sure whether you should use Bador, ask your doctor or pharmacist.
Take special care with Bador
Tell your doctor about any health problems that you currently have or have had in the past particularly:
• liver problems
• any allergies
• eye problems and eye surgeries
Contact your doctor immediately if you have any eye problems, such as:
• eye irritation
• any other eye problems, such as eye redness or swollen surface layer of the eye or eyelids
• an eye infection
• an eye injury
• you are undergoing eye surgery
• you have any new or worsening symptoms.
Use of Bador in the eye can affect the whole body.
If you develop allergic reactions including hives (nettle rash), skin rash, eye redness and itching, swelling of the face, lips, tongue and/or throat, which may cause difficulties in breathing or swallowing, stop using this medicine and seek immediate medical advice.
If you wear soft contact lenses, consult your doctor before using Bador. See also 'contact lenses' at the end of section 2.
Use in children
Bador has been studied in infants and children less than six years of age who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Use in elderly patients
The effect of Bador on the elderly is similar to all other adult patients.
Using other medicines
Please tell your doctor or pharmacist if you are taking/using or have recently taken/used any other medicines, including other eye drops or medicines obtained without a prescription.
This is particularly important if you have liver problems.
This is particularly important if you are taking/using any of the following:
• other carbonic anhydrase inhibitors (e.g. acetazolamide), taking by mouth, as eye drops, or by some other method
• large doses of acetylsalicylic acid (e.g. aspirin)
Pregnancy and breast-feeding
Pregnancy
You should not use Bador during pregnancy.
Tell your doctor if you are pregnant or planning to become pregnant.
Breast-feeding
You should not use Bador while breast-feeding.
Tell your doctor if you are breast-feeding or intending to breast-feed.
Driving and using machines
During Bador treatment, possible side effects such as dizziness and blurred vision may affect your ability to drive and/or use machines. Do not drive and do not operate any tools or machinery until you feel better or your vision has cleared.
Important information about some of the ingredients of Bador
Bador contains benzalkonium chloride as a preservative, which may cause eye irritation.
Contact lenses
If you wear contact lenses, you should consult your doctor before using this medicine.
Contact lenses should be removed prior to use of the drops and should not be reinserted for at least 15 minutes post-administration. The preservative in Bador (benzalkonium chloride) leads to discolouration of soft contact lenses.
3. HOW TO USE BADOR
Always use Bador exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Your doctor will decide on the proper dose and length of treatment.
Dosage
Using Bador on its own
The usual dose is 1 Bador drop into each affected eye 3 times a day, for example in the morning, in the afternoon and in the evening.
Using Bador alongside other eye drops
If your doctor has recommended you use Bador with a beta-blocker eye drop to lower eye pressure, then the usual dose is 1 drop of Bador in the affected eye(s) 2 times a day, for example in the morning and in the evening. If you are using Bador together with other eye drops, the drops should be administered at least 10 minutes apart.
Replacing another eye drop with Bador
If you are going to use Bador to replace another eye drop medicine used to lower eye pressure, you should use your other eye drops as usual on one day and start Bador on the next day.
If you are confused by this then talk to your doctor who will explain this to you.
Do not change the prescribed dose of this medicine without asking your doctor first.
Avoiding eye infections
Do not touch your eyes - or the area around the eyes - with the dropper tip of the container. The eye drops may otherwise become contaminated with bacteria, which could lead to an eye infection, resulting in serious eye damage and even loss of vision. To avoid contamination of the container, wash your hands before using this medicine and prevent the tip of the container from coming into contact with any surfaces. If you think that your medicine is contaminated or if you develop an eye infection, contact your doctor immediately regarding further use of this bottle.
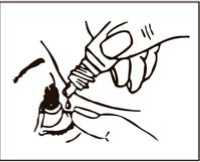
Instructions for proper use
1. Wash your hands and sit or stand comfortably.
2. Twist off the cap.
3. Tilt your head backwards and use your finger to gently pull down the lower eyelid of your affected eye.
4. Place the tip of the bottle close to, but not touching your eye.
5. Squeeze the bottle gently so that only one drop goes into your eye, then release the lower eyelid.
6. Repeat in your other eye if your doctor has told you to do this.
7. Put the cap back on the bottle.
If you use more Bador than you should
If you have put too many drops into your eye or have swallowed some of the container contents, you should contact your doctor immediately. You may - among other things - start to feel sleepiness, nausea, dizziness, headache, tiredness, abnormal dreams and difficulty to swallow.
If you forget to use Bador
It is important that you use Bador as directed by your doctor. If you forget a dose, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and resume your regular dosing schedule. Do not use a double dose to make up for a forgotten dose.
If you stop using Bador
If you would like to stop treatment with this medicine, talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Bador can cause side effects, although not everybody gets them. If they do occur, you might need medical care.
Allergic reactions
If you develop allergic reactions including hives (nettle rash), skin rash, eye redness and itching, swelling of the face, lips, tongue and/or throat, which may cause difficulties in breathing or swallowing, stop using this medicine and seek immediate medical advice.
The following side effects have been reported with Bador or either of its active substances.
Very Common side effects: (more than 1 user in 10)
Burning and stinging of the eyes.
Common side effects: (l to 10 users in 100)
Disease of the cornea with sore eye and blurred vision (superficial punctuate keratitis), discharge with itching of the eyes (conjunctivitis), irritation/inflammation of the eyelid, blurred vision, headache, nausea, bitter taste, and fatigue.
Uncommon side effects: (l to 10 users in 1,000)
Inflammation of the iris.
Rare side effects:(1 to 10 users in 10,000)
Tingling or numbness of the hands or feet, temporary shortsightedness which may resolve when treatment is stopped, development of fluid under the retina (choroidal detachment, following filtration surgery), eye pain, eyelid crusting, low pressure in the eye, swelling of the cornea (with symptoms of visual disturbances), eye irritation including redness, kidney stones, dizziness, nose bleed, throat irritation, dry mouth, localized skin rash (contact dermatitis), allergic type reactions such as rash, hives, itching, in rare cases possible swelling of the lips, eyes and mouth, shortness of breath, and more rarely wheezing, severe skin rash.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE BADOR
Keep out of the reach and sight of children.
Do not use Bador after the expiry date stated on the container (after “EXP”). The expiry date refers to the last day of that month.
Do not store above 25°C. Keep the bottle in the outer carton in order to protect from light.
You can use Bador for up to 28 days after opening the bottle.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Bador contains
• The active substance is dorzolamide.
• Each ml contains 20 mg dorzolamide (as dorzolamide hydrochloride)
• The other ingredients are hydroxyethyl cellulose, mannitol (E421), sodium citrate, sodium hydroxide and water for injection. Benzalkonium chloride (0.075mg/ml) is added as a preservative.
What Bador looks like and contents of the pack
Bador is a clear, nearly colourless, sterile eye drop solution.
LDPE bottle with LLDPE dropper tip and PP cap containing 5 ml solution. Pack sizes
1 x 5 ml (one bottle containing 5 ml eye drops)
3 x 5 ml (three bottles, each containing 5 ml eye drops)
6 x 5 ml (six bottles, each containing 5 ml eye drops)
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Bausch & Lomb UK Ltd 106 London Road Kingston-Upon-Thames Surrey KT2 6TN UK
Manufacturer
Dr. Gerhard Mann Chem.-pharm. Fabrik GmbH Brunsbutteler Damm 165 - 173 13581 Berlin Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
<{Name of the Member State}> <{Name of the medicinal product}>
This leaflet was last approved in 09/2011.
6