Balance 2.3% Glucose 1.25Mmol/L Calcium Solution For Peritoneal Dialysis
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
balance 2.3% glucose, 1.25 mmol/l calcium, solution for peritoneal dialysis
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
balance 2.3% glucose, 1.25 mmol/l calcium is delivered in a double chamber bag. One chamber contains the alkaline lactate solution, the other chamber contains the acidic glucose-based electrolyte solution. Mixing of both solutions by opening the middle seam between the two chambers results in the neutral ready-to-use solution.
BEFORE RECONSTITUTION
1 litre of acidic glucose based electrolyte solution contains:
Sodium chloride 11.279 g
Calcium chloride dihydrate 0.3675 g
Magnesium chloride hexahydrate 0.2033 g Glucose, anhydrous 45.46 g
(as glucose monohydrate) 1 litre of alkaline lactate solution contains:
Sodium lactate 7.85 g
(as sodium lactate solution)
AFTER RECONSTITUTION
1 litre of the neutral ready-to-use solution contains:
|
Sodium chloride |
5.640 |
g |
|
Sodium lactate |
3.925 |
g |
|
(as sodium lactate solution) | ||
|
Calcium chloride dihydrate |
0.1838 |
g |
|
Magnesium chloride hexahydrate |
0.1017 |
g |
|
Glucose, anhydrous |
22.73 |
g |
(as glucose monohydrate)
|
Na+ |
134 |
mmol/l |
|
Ca++ |
1.25 |
mmol/l |
|
Mg++ |
0.5 |
mmol/l |
|
Cl- |
100.5 |
mmol/l |
|
Lactate |
35 |
mmol/l |
|
Glucose |
126.1 |
mmol/l |
|
Theoretical | ||
|
osmolarity |
399 |
mOsm/l |
|
pH - |
7.0 | |
For a full list of excipients, see section 6.1
3. PHARMACEUTICAL FORM Solution for peritoneal dialysis
Double chamber bag containing clear and colourless aqueous solutions
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
End-stage (decompensated) chronic renal failure of any origin treated with peritoneal dialysis.
4.2 Posology and method of administration
Dosage
This solution is indicated exclusively for intraperitoneal use.
The mode of therapy, frequency of administration, and dwell time required will be specified by the attending physician.
Unless otherwise advised, patients will receive an infusion of 2000 ml solution per exchange four times a day. After a dwell time between 2 and 10 hours the solution will be drained.
Adjustment of dosage, volume and number of exchanges will be necessary for individual patients.
If pain due to abdominal distension occurs at the commencement of peritoneal dialysis, the solution volume per exchange should be temporarily reduced to 500-1500 ml.
In large patients, and if residual renal function is lost, an increased volume of dialysis solution will be necessary. In these patients, or patients who tolerate larger volumes, a volume of 2500-3000 ml solution per exchange may be given.
If a machine is used for intermittent or continuous cyclic peritoneal dialysis, the use of larger bags is recommended providing more than one solution exchange.
In children the solution volume per exchange should be reduced according to age, height and body weight (30-40 ml/kg body weight).
There are no special dosage recommendations for elderly patients.
Peritoneal dialysis solutions with a high glucose concentration (2.3% or 4.25%) are used when the body weight is above the desired dry weight. The withdrawal of fluid from the body increases in relation to the glucose concentration of the peritoneal dialysis solution. These solutions should be used cautiously to handle the peritoneal membrane with care, to prevent dehydration and in order to keep the glucose burden as low as possible.
Dialysis using the prescribed doses should be performed daily. Peritoneal dialysis is a long-term therapy involving repeated administrations of single solutions.
balance 2.3% glucose, 1.25 mmol/l calcium contains 22.73 g glucose in 1000 ml solution.
Method and duration of administration
For stay safe balance, the solution bag is first warmed up to body temperature. The heating will be performed with a heating plate. The time for heating is about 120 minutes for a 2000 ml bag at a temperature of 22°C. Details can be read in the instruction manual of the heating plate. A microwave oven must not be used due to the risk of local overheating.
The solutions in the two chambers must be mixed before use. For the step-by-step instruction for use please see Section 6.6.
Depending on physician's instructions, the dose should dwell in the peritoneal cavity for 2 to 10 hours (equilibrium time), and then be drained. Depending on the required osmotic pressure, balance 2.3% glucose, 1.25 mmol/l calcium can be used sequentially with other peritoneal dialysis solutions with lower or higher glucose content (i.e. with lower or higher osmolarity).
Before performing peritoneal dialysis at home the patient must be trained appropriately, must practice the technique and be shown to be proficient. The training should be performed by qualified personnel. The attending physician must ensure that the patient masters the handling techniques sufficiently before being discharged to carry out peritoneal dialysis at home. In case of any problems or uncertainty the attending physician should be contacted.
Peritoneal dialysis should be continued for as long as renal function substitution therapy is required.
4.3 Contraindications
For this specific peritoneal dialysis solution
balance 2.3% glucose, 1.25 mmol/l calcium must not be used in patients with severe hypokalaemia and severe hypocalcaemia.
For peritoneal dialysis in general
Peritoneal dialysis should not be commenced if any of the following are present:
- recent abdominal surgery or injury, a history of abdominal operations with fibrous adhesions, abdominal burns, bowel perforation,
- extensive inflammatory conditions of the abdominal skin (dermatitis),
- inflammatory bowel diseases (Crohn's disease, ulcerative colitis, diverticulitis),
- peritonitis,
- internal or external abdominal fistula,
- umbilical, inguinal or other abdominal hernia,
- intra-abdominal tumours,
- ileus,
- pulmonary disease (especially pneumonia),
- sepsis,
- lactic acidosis,
- extreme hyperlipidaemia,
- in rare cases of uraemia, which can not be managed by peritoneal dialysis,
- cachexia and severe weight loss, particularly in cases where ingestion of adequate protein is not guaranteed,
- patients who are physically or mentally incapable of performing peritoneal dialysis as instructed by the physician.
If any of the above mentioned disorders develops during the peritoneal dialysis, the attending physician will have to decide on how to proceed.
4.4 Special warnings and precautions for use
This solution may only be administered after careful benefit-risk assessment
in:
• loss of electrolytes due to vomiting and/or diarrhoea (a temporary change to a peritoneal dialysis solution containing potassium might then become necessary).
• patients with hyperparathyroidism: Therapy should include the administration of calcium
• hypocalcaemia: It may be necessary to use a peritoneal dialysis solution with a higher calcium concentration either temporarily or permanently, in case an adequate enteral supply of calcium, by calcium-containing phosphate binders and/or vitamin D, is not possible.
• patients receiving digitalis therapy: Regular monitoring of the serum potassium level is
• patients with large polycystic kidneys.
The effluent should be checked for clarity and volume. Turbidity, which may or may not be accompanied by abdominal pain, or abdominal pain alone are indicators of peritonitis.
A loss of proteins, amino acids, and water-soluble vitamins occurs during peritoneal dialysis. To avoid deficiencies an adequate diet or supplementation should be ensured.
The transport characteristics of the peritoneal membrane may change during long-term peritoneal dialysis primarily indicated by a loss of ultrafiltration. In severe cases peritoneal dialysis must be stopped and haemodialysis commenced.
Regular monitoring of the following parameters is recommended:
- body weight for the early recognition of over- and dehydration,
- serum sodium, potassium, calcium, magnesium, phosphate, acid base status, blood gases and blood proteins,
- serum creatinine and urea,
- parathormone and other indicators of bone metabolism,
- blood sugar,
- residual renal function in order to adapt the peritoneal dialysis.
Elderly patients
The increased incidence of hernia should be considered in elderly patients prior to the start of peritoneal dialysis.
Shelf life of the ready-to-use solution
The ready-to-use solution should be used immediately, but within a maximum of 24 hours after mixing (see also Section 6.3).
Handling (see also Section 6.6)
Plastic containers may occasionally be damaged during transport or storage. This can result in contamination with growth of microorganisms in the dialysis solution. Thus all containers should be carefully inspected for damage prior to connection of the bag and prior to use of the peritoneal dialysis solution. Any damage, even minor, to connectors, at the closure, container welds and corners must be noted because of possible contamination. Damaged bags or bags with cloudy content should never be used.
This solution must only be used if the solution for dialysis is clear and the container undamaged. Any unused portion of the solution is to be discarded.
The overwrap should only be removed before administration.
Do not use before mixing the two solutions.
Aseptic conditions must be maintained during dialysate exchange in order to reduce the risk of infection.
4.5 Interaction with other medicinal products and other forms of interaction
The use of this peritoneal dialysis solution can lead to a loss of efficacy of other medicinal products if these are dialysable through the peritoneal membrane. A dose adjustment might be necessary.
A distinct reduction of the serum potassium level can increase the frequency of digitalis-associated adverse reactions. Potassium levels must be monitored particularly closely during concurrent digitalis therapy.
The use of diuretic agents may help maintain residual diuresis, but may also result in water and electrolyte imbalances.
In diabetic patients the daily dose of insulin or oral hypoglycaemic medicinal products must be adjusted to take account of the increased glucose load.
4.6 Pregnancy and lactation
There are no adequate data from use of balance solutions in animal studies or in pregnant women.
Caution should be exercised when prescribing to pregnant women or during the lactation period.
4.7 Effects on ability to drive and use machines
balance 2.3% glucose, 1.25 mmol/l calcium has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
balance 2.3% glucose, 1.25 mmol/l calcium is an electrolyte solution the composition of which is similar to blood. In addition the solution has a neutral pH which is similar to the physiological pH value.
Possible adverse reactions may result from the peritoneal dialysis itself or may be induced by the peritoneal dialysis solution.
Potential adverse reactions of the peritoneal dialysis solution
Endocrine disorders
Secondary hyperparathyroidism with potential disturbances of the bone metabolism.
Metabolism and nutrition disorders
Increased blood sugar levels; hyperlipidaemia; increase in body weight due to the continuous uptake of glucose from the peritoneal dialysis solution.
Cardiac and vascular disorders
Tachycardia; hypotension; hypertension.
Respiratory, thoracic and mediastinal disorders Dyspnoea.
Renal and urinary disorders
Electrolyte disturbances, e.g. hypokalaemia (very common (> 10%)), hypocalcaemia.
General disorders
Dizziness; oedema; disturbances in hydration indicated either by a rapid decrease (dehydration) or increase (overhydration) in body weight. Severe dehydration might occur when using solutions of higher glucose concentration.
Potential adverse reactions of the treatment mode
Infections and infestations
Peritonitis (very common (> 10%)); skin exit site and tunnel infections (very common (> 10%)); in very rare cases sepsis (< 0.01%).
Respiratory, thoracic and mediastinal disorders Dyspnoea caused by the elevated diaphragm; shoulder pain.
Gastrointestinal disorders
Diarrhoea; constipation; hernia (very common (> 10%)); abdominal distension and sensation of fullness.
General disorders and administration/catheter site conditions
General malaise; redness, oedema, exudations, crusts and pain at the catheter
exit site.
Peritoneal dialysis procedure related disorders
Cloudy effluent; in- and outflow disturbances of the dialysis solution.
Peritonitis is indicated by a cloudy effluent. Later abdominal pain, fever, and general malaise may develop or, in very rare cases, sepsis. The patient should seek medical advice immediately. The bag with the cloudy effluent should be closed with a sterile cap and assessed for microbiological contamination and white blood cell count.
In case of skin exit site and tunnel infections the attending physician should be consulted as soon as possible.
4.9 Overdose
No emergency situations in connection with overdose have been reported. Excessive inflow of dialysis solution is easily drained into an empty bag.
However, if bag exchanges have been carried out too frequently or too rapidly, states of dehydration and/or electrolyte disturbances can occur which necessitate immediate medical attention. If an exchange has been forgotten, then as a rule the dwell times of the next bags should be reduced in such a way that the total amount of dialysis solution required per day (e.g. 4 x 2000 ml) is achieved.
Incorrect balancing can lead to hyper- or dehydration and electrolyte disturbances.
The most likely consequence of an overdosage with balance 2.3% glucose,
1.25 mmol/l calcium is dehydration. Underdosage, interruption of treatment or discontinuation of treatment may lead to life-threatening hyperhydration with peripheral oedema and cardiac decompensation and/or other symptoms of uraemia, which may endanger life.
The generally accepted rules for emergency care and intensive therapy must be applied. The patient may require immediate haemodialysis.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Solution for peritoneal dialysis ATC code: B05D B
balance 2.3% glucose, 1.25 mmol/l calcium is a lactate-buffered, glucose-containing electrolyte solution indicated for intraperitoneal administration for the treatment of end-stage renal failure of any origin.
The characteristic of continuous ambulatory peritoneal dialysis (CAPD) is the more or less continuous presence of usually 2 litres of dialysis solution in the peritoneal cavity. This dialysis solution is replaced by fresh solution three to five times a day.
The basic principle behind every peritoneal dialysis technique is the use of the peritoneum as a semi-permeable membrane allowing the exchange of solutes and water between the blood and the dialysis solution by diffusion and convection according to their physico-chemical properties.
The electrolyte profile of the solution is basically the same as that of physiological serum, although it has been adapted (e.g. the potassium content) for use in uraemic patients to enable renal function substitution therapy by means of intraperitoneal substances and fluid exchange. The calcium concentration of this dialysis solution is 1.25 mmol/l, which has been shown to reduce the risk of hypercalcaemia during concomitant treatment with calcium containing phosphate binders and/or vitamin D. Substances which are normally eliminated with the urine, like uraemic waste products, such as urea and creatinine, inorganic phosphate, uric acid, other solutes and water, are removed from the body into the dialysis solution. The fluid balance can be maintained by the administration of different glucose-concentrations in the solution, effecting the fluid removal (ultrafiltration).
Metabolic acidosis secondary to end-stage renal failure is counterbalanced by the presence of lactate in the solution. The complete metabolism of lactate results in the generation of bicarbonate.
5.2 Pharmacokinetic properties
Uraemic waste products (e.g. urea, creatinine, uric acid), inorganic phosphate and electrolytes like sodium, potassium, calcium and magnesium are removed from the body to the dialysis solution by diffusion and/or convection.
Glucose in the dialysate is used as an osmotic agent in balance 2.3% glucose, 1.25 mmol/l calcium. It is slowly absorbed, reducing the diffusion gradient between dialysis solution and extracellular fluid. The ultrafiltration is maximal at the beginning of the dwell time, reaching a peak after about two to three hours. Later absorption starts with a progressive loss of ultrafiltrate. After 4 hours the ultrafiltrate averages 100 ml with a 1.5%, 400 ml with a 2.3%, and 800 ml with a 4.25% glucose solution. During the dialysis period of six hours 60 to 80% of dialysate glucose is absorbed.
Lactate used as the buffering agent is almost completely absorbed after a 6 hour dwell time. In patients with a normal hepatic function lactate is rapidly metabolised demonstrated by normal values of intermediate metabolites.
The transfer of calcium depends on the glucose concentration in the dialysis solution, the effluent volume, the serum ionised calcium and the calcium concentration in the dialysis solution. The higher the glucose concentration, effluent volume and serum calcium concentration, and the lower the calcium concentration in the dialysis solution, the higher is the calcium transfer from the patient to the dialysate.
It has been estimated that a typical CAPD schedule of three 1.5% and one 4.25 % glucose containing solutions per day with a calcium concentration of 1.25 mmol/l would remove up to 160 mg calcium per day enabling a higher oral intake of calcium containing drugs and vitamin D without the risk of hypercalcaemia.
5.3 Preclinical safety data
No preclinical toxicity studies with balance 2.3% glucose, 1.25 mmol/l calcium have been carried out, but clinical studies with comparable solutions for peritoneal dialysis have shown no major risk of toxicity.
PHARMACEUTICAL PARTICULARS
6.
6.1 List of excipients
Water for injections Hydrochloric acid Sodium hydroxide Sodium hydrogen carbonate
6.2 Incompatibilities
Because of the risk of incompatibility and of contamination medicinal products must only be added when prescribed by a physician.
This medicinal product should not be used with other medicinal products except those mentioned in Section 6.6.
6.3 Shelf life
Shelf life as packed for sale: 2 years.
Shelf life after mixing: Chemical and physical in-use stability has been demonstrated for 24 hours at 20°C.
6.4 Special precautions for storage
Do not store below 4°C
6. 5 Nature and contents of container
Double chamber bag:
Lactate solution: glucose-based electrolyte solution = 1:1
Double chamber bag system consisting of a non-PVC double chamber solution bag which is wrapped in a protective bag, both made of multi layer polyolefine foils.
There are two versions of the container available as follows:
The stay safe balance system contains the double chamber bag system, a tubing system made of polyolefines, a system connector (DISC) with a rotateable switch (polypropylene) and a drainage bag, also made of polyolefine multi layer film.
The sleep safe balance system contains the double chamber bag system and a bag connector which consists of polypropylene.
Pack sizes: stay safe balance
4 bags each containing 1500 ml 4 bags each containing 2000 ml 4 bags each containing 2500 ml 4 bags each containing 3000 ml
sleep safe balance
4 bags each containing 3000 ml
Not all pack sizes may be marketed.
6.5 Nature and contents of container
Double chamber bag:
Lactate solution : glucose-based electrolyte solution = 1:1
Double chamber bag system consisting of a non-PVC double chamber solution bag which is wrapped in a protective bag, both made of multi layer polyolefine foils.
There are three versions of the container available as follows:
The stay safe balance system contains the double chamber bag system, a tubing system made of polyolefines, a system connector (DISC) with a rotable switch (polypropylene) and a drainage bag, also made of polyolefine multi layer film.
The sleep safe balance system contains the double chamber bag system and a bag connector which consists of polypropylene.
The safe lock balance system contains the double chamber bag system and a safe lock connector which consists of polycarbonate.
Pack sizes:
stay safe balance
4 bags each containing 1500 ml 4 bags each containing 2000 ml 4 bags each containing 2500 ml 4 bags each containing 3000 ml
sleep safe balance
4 bags each containing 3000 ml 2 bags each containing 5000 ml 2 bags each containing 6000 ml
safe lock balance
2 bags each containing 5000 ml 2 bags each containing 6000 ml
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
For single use only. Any unused portion of the solution is to be discarded.
For stay safe balance, first warm up the solution bag to body temperature. For bags with a volume up to 3000 ml this should be done using an appropriate heater tray. The heating time for a 2000 ml bag with a starting temperature of 22°C is approx. 120 min. The temperature control is done automatically and is set to 39°C ± 1°C. More detailed information can be obtained from the operating instructions of the bag warmer. Use of microwaves is not recommended due the risk of local overheating. sleep safe balance and safe lock balance bags are applied in combination with a cycler. Warming up is performed automatically by the heater of the cycler. After warming the solution you can start with the exchange of the bags.
Instruction for use of the stay safe balance system:
1. Preparation of the solution
• Check the warmed solution bag (label, expiry date, clearness of the solution, bag and overwrap not damaged, integrity of peel seams).
• Place the bag on a solid surface.
• Open the overwrap of the bag and the packaging of the disinfection cap.
• Wash your hands with an antimicrobial washing lotion.
• Roll up the bag, which is lying on the outer wrapping foil, from one of the side edges until the middle seam opens. The solutions in the two chambers are mixed automatically.
• Now roll up the bag from the upper edge until the peel seam of the lower triangle is completely open.
• Check whether all peel seams are completely open.
• Check whether the solution is clear and that the bag is not leaking.
2. Preparation of the bag exchange
• Hang the solution bag in the upper hole of the infusion pole, unroll the tubing line of the solution bag, and place the DISC into the organizer. After unrolling the tubing line to the drainage bag, hang the drainage bag in the lower hole of the infusion pole and place the disinfection cap into the organizer.
• Place catheter connector into the organizer.
• Disinfect your hands and remove the protection cap of the DISC.
• Connect catheter connector to the DISC.
3. Outflow
• Open the catheter extension clamp. The outflow starts.
^ Position (
4. Flush
• After completion of outflow fill the tube between solution bag and DISC completely with fluid by flushing fresh solution into the drainage bag (approx. 5 seconds).
^ Position ((
5. Inflow
• Start inflow by turning the control switch to ^ Position *)(
6. Safety step
• Close the catheter extension by introducing the Pin into the catheter connector.
^ Position ((((
7. Disconnection
• Remove protection cap from the new disinfection cap and screw it to the old one.
• Screw the catheter connector off the DISC and screw the catheter connector to the new disinfection cap.
8. Closure of the DISC
• Close the DISC with the open end of the protection cap of the used disinfection cap, which is placed in the right hole of the organizer.
9. Check the drained dialysate for clarity and weight and if the effluent is clear discard it.
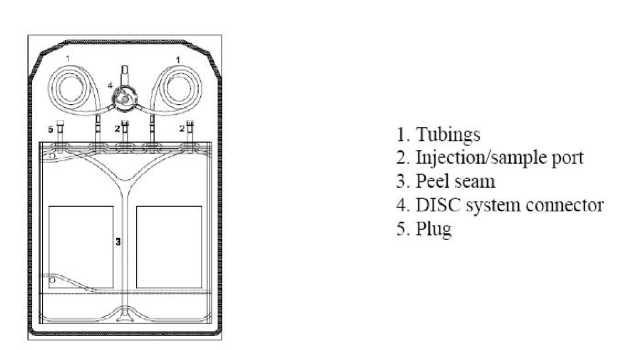
Instruction for use of the 3000 ml sleep safe balance system:
1. Preparation of the solution
• Check the solution bag (label, expiry date, clearness of the solution, bag and overwrap not damaged, integrity of peel seams).
• Place the bag on a solid surface.
• Open the overwrap of the bag.
• Wash your hands with an antimicrobial washing lotion.
• Roll up the bag, which is lying on the outer wrapping foil, from one of the side edges until the middle seam opens. The solutions in the two chambers are mixed automatically.
• Now roll up the bag from the upper edge until the peel seam of the lower triangle is completely open.
• Check whether all peel seams are completely open.
• Check whether the solution is clear and that the bag is not leaking.
2. Unroll tubing (1) of bag.
3. Remove the protection cap.
4. Insert bag connector in free tray port of the sleep safe cycler.
5. The bag is now ready for use with the sleep safe set.
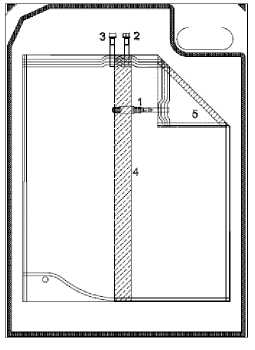
Instruction for use of the 5000 ml and 6000 ml sleep safe balance system:
1. Preparation of the solution
• Check the solution bag (label, expiry date, clearness of the solution, bag, and overwrap not damaged, peel seams intact).
• Place the bag on a solid surface.
• Open the overwrap of the bag.
• Wash your hands with an antimicrobial washing lotion.
• Unfold middle peel seam and bag connector.
• Roll up the bag, which is lying on the overwrap, from the diagonal end towards the bag connector. The middle peel seam (4) will open.
• Continue until the peel seam of the small chamber (5) opens as well.
• Check that all peel seams are completely open.
• Check that the solution is clear and that the bag is not leaking.
2. Unroll tubing (1) of bag.
3. Remove the protection cap.
4. Insert bag connector in free tray port of the sleep safe cycler.
5. The bag is now ready for use with the sleep safe set.
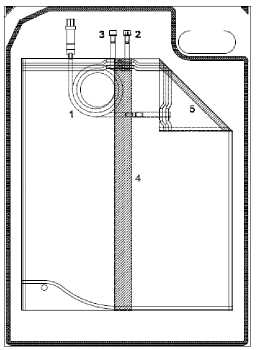
1. Tubing with bag connector
2. Injection port
3. Plug
4. Middle peel seam
5. Peel seam of small chamber
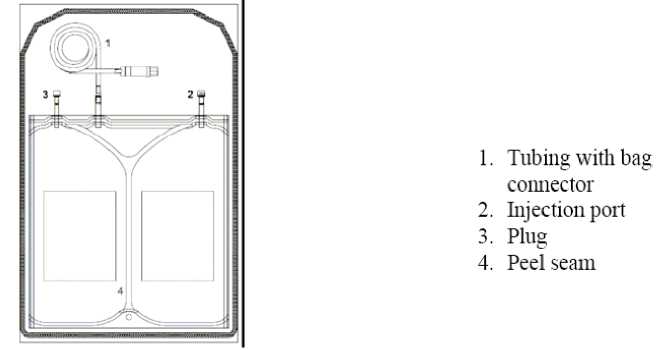
Instruction for use of the safe lock balance system:
1. Preparation of the solution
• Check the solution bag (label, expiry date, clearness of the solution, bag, and overwrap not damaged, peel seams intact).
• Place the bag on a solid surface.
• Open the overwrap of the bag.
• Wash your hands with an antimicrobial washing lotion.
• Unfold middle peel seam and bag connector.
• Roll up the bag, which is lying on the overwrap, from the diagonal end towards the bag connector. The middle peel seam (4) will open.
• Continue until the peel seam of the small chamber (5) opens as well.
• Check that all peel seams are completely open.
• Check that the solution is clear and that the bag is not leaking.
2. Remove protective cap of the connector (1) from the connecting line.
3. Connect lines to the bag.
4. Break the inner lock by bending the line and the pin by more than 90° to both sides.
5. The bag is now ready for use.
|
1. |
Safe lock connector |
|
2. |
Injection port |
|
3. |
Plug |
|
4. |
Middle peel seam |
|
5. |
Peel seam of small |
|
chamber |
General instructions applicable to all application systems:
The ready-to-use solution should be used immediately, but if this is not possible within a maximum of 24 hours after mixing (see also Section 6.3).
If prescribed, medicinal products may be added to the ready-to-use solution through the injection port (2) taking great care not to introduce any contamination.
Only the following medicinal products may be added up to the mentioned concentration if indicated by the attending physician: Heparin 1000 I.U./l, insulin 20 I.U./l, vancomycin 1000 mg/l, teicoplanin 400 mg/l, cefazolin 500 mg/l, ceftazidime 250 mg/l, gentamicin 8 mg/l. After thorough mixing and checking for the absence of any turbidity or particles the peritoneal dialysis solution must be used immediately (no storage).
Fresenius Medical Care Deutschland GmbH
D-61346 Bad Homburg
Germany
8. MARKETING AUTHORISATION NUMBER
PL 13689/0013
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
17/11/2009
10 DATE OF REVISION OF THE TEXT
17/11/2009