Budesonide 64 Micrograms/Actuation Aqueous Nasal Spray
PACKAGE LEAFLET: INFORMATION FOR THE USER
SZ00000LT000
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Budesonide Nasal Spray is and what it is used for
2. What you need to know before you take Budesonide Nasal Spray
3. How to take Budesonide Nasal Spray
4. Possible side effects
5. How to store Budesonide Nasal Spray
6. Contents of the pack and other information
What Budesonide Nasal 1 Spray is and what it is used for
Budesonide Nasal Spray contains budesonide, a synthetic corticosteroid. Corticosteroids are a group of medicines which help fight inflammation. Budesonide Nasal Spray is used for:
• the treatment and prevention of the symptoms of allergies like hay fever (e.g. caused by grass pollen)
• the treatment and prevention of the symptoms of year-round nasal allergy caused for example by house dust (chronic rhinitis)
• the treatment of symptoms of nasal polyps (small growths on the lining of the nose).
Driving and using machines
This medicine does not affect your ability to drive and use machines at the recommended dose (see section 3. How to use Budesonide Nasal Spray).
Budesonide Nasal Spray contains potassium sorbate
Potassium sorbate is an ingredient of Budesonide Nasal Spray. It may cause irritation of the skin or mucous membranes (e.g. contact dermatitis).
How to take Budesonide Nasal Spray
What you need to know 2 before you take Budesonide Nasal Spray
Do not take Budesonide Nasal Spray
• if you are allergic to budesonide, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before taking Budesonide Nasal Spray
• if you have been taking this medicine continuously for a long time, your doctor will want to examine the inside of your nose at least every six months.
• if you have been taking higher than recommended doses of this medicine: Your doctor may prescribe steroid tablets during periods of stress (such as if you have an infection) or before an operation.
• if you have any ulcers in your nose.
• if your have infectious blisters on your lips (a cold sore), in the nose or around your eyes.
• if you suffer from nosebleeds.
• if you have had an operation on your nose or any other injury to your nose that has not yet fully recovered.
• if you have a bacterial, viral or fungal infection of your nose: You should use Budesonide Nasal Spray if you were prescribed treatment for the infection as well.
• if you have problems with your liver because the amount of budesonide could build up in your body. Your doctor might need to check your liver, and as a result, may need to reduce your dose.
• if you were switched from another dosage form to the nasal spray and have problems with your adrenal function.
• if you were told by your doctor you have infection of airways or lung tuberculosis. This is an infection that can affect your lungs.
Children
• if you are a child and are taking
high doses of this medicine for a long time, your doctor will check your height regularly
Other medicines and Budesonide Nasal Spray
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Make sure you tell your doctor if you are taking ketoconazole tablets, a medicine used to treat fungal infections, such as thrush. It may increase the concentration of budesonide in your body.
You should also tell your doctor if you are taking any other medicines such as:
• troleandomycin, a medicine to treat bacterial infections
• itraconazole, a medicine to treat fungal infections
• ciclosporin, immune suppression drug used e.g. in connection with transplants
• ethinylestradiol, used for contraception. It is possible that these medicines could also increase the concentration of budesonide in your body.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should not use Budesonide nasal spray if you are pregnant unless you have discussed this with your doctor.
Breast-feeding mothers can use
Budesonide nasal spray, but only if the doctor decides that the benefits for the mother are weighted against the risk for the suckling child. Be sure to tell your doctor immediately if you are breast-feeding.
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Budesonide Nasal Spray is intended for nasal use. It should be sprayed into your nostrils as described below.
Dosage
The dose should be adjusted to suit you. Use the lowest dose which still relieves your symptoms.
Allergic rhinitis
Starting dose
Adults, adolescents (12 years of age and older) and children older than 6 years of age:
The recommended starting dose of Budesonide Nasal Spray is a total of 4 sprays (256 micrograms) each day.
You can either use this medicine:
• once daily by applying 2 sprays into each nostril in the morning
or
• twice daily by applying 1 spray into each nostril in the morning and 1 spray into each nostril in the evening.
Children should be treated under guidance of an adult.
Ideally, you should start to use this medicine up to 14 days before you expect your symptoms to start. For example, if you have hay fever, start using this medicine about 2 weeks before your hay fever symptoms usually begin to be a problem and stop using this medicine after the end of season of allergen exposure.
Maintenance dose
It takes 7 to 14 days for this medicine to work. After this, your doctor may lower your dose.
Nasal polyps
Adults, adolescents (12 years of age and older) and children older than 6 years of age:
The recommended starting dose of Budesonide Nasal Spray is a total of 4 sprays (256 micrograms) each day.
You can either use this medicine:
• once daily by applying 2 sprays into each nostril in the morning
or
• twice daily by applying 1 spray into each nostril in the morning and 1 spray into each nostril in the evening.
Children should be treated under guidance of an adult.
Once the effect is achieved, the lowest dose which relieves your symptoms should be used.
Using more than the recommended 4 sprays of this medicine each day will not make this medicine work any better.
Duration of treatment:
Your doctor will tell how long your treatment with Budesonide Nasal Spray will last. You must use this medicine regularly or it will not work properly. Do not stop treatment even if you feel better unless told to by your doctor.
If you don't experience an immediate relief, you should continue using your medicine regularly as it may take a few days to start working.
Method of administration
1. Gently blow your nose to clean the nostrils, if necessary.
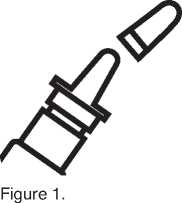
2. Shake the bottle. Remove the protective cap (figure 1).
Continued on the next page >>
|
Artwork Proof Box Ref: V013 - Art. 45 Paediatric WS (NL/W/0001/pdWS/001) | ||
|
Proof no. |
Date prepared: |
Font size: |
|
004.0 |
16/05/2014 |
8pt |
|
Colours: |
Fonts: | |
|
■ Black |
□ |
Helvetica |
|
□ |
□ | |
|
Dimensions: |
130 x 540 mm |
_y |
3. Hold the bottle as shown in figure 2. Before using Budesonide Nasal Spray for the first time you must prime the nozzle (i.e. fill it with medicine). Pump the nozzle up and down several times (5-10 times), spraying into the air until an even mist is seen. The priming effect remains for approximately 24 hours. If a longer period of time passes before the next dose is taken, the nozzle must be primed (filled with medicine) again. If Budesonide Nasal Spray is used at shorter intervals it is sufficient to spray just once into the air.
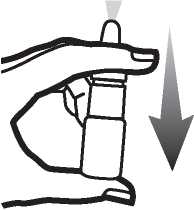
Figure 2.
4. Insert the tip of the nozzle into your nostril as shown in figure 3. Spray once (or more if your doctor has told you to). Spray into the other nostril in the same way. Note, it is not necessary to breathe in at the same time as you spray.
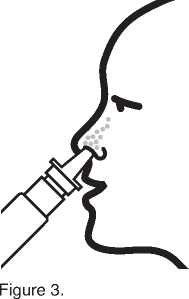
5. Wipe the nozzle with a clean tissue and replace the protective cap.
6. Store the bottle in an upright position.
Cleaning your Budesonide Nasal Spray You should clean the plastic nozzle of Budesonide Nasal Spray regularly, and at any time the spray of medicine is not coming out as it should. If this happens, first check if the nozzle is primed with medicine (see earlier). If after priming the nozzle again the pump is still not working, clean the nozzle by using the following instructions:
• Remove the plastic nozzle with a clean tissue and wash in warm - not hot -water.
• Rinse the nozzle thoroughly, dry it and then replace onto the top of the bottle.
• Never try to unblock the nozzle by using a pin or other sharp object.
• After cleaning, the nozzle must be primed (filled with medicine) again before use.
If you use more Budesonide Nasal Spray than you should
It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should use only as much as your doctor recommends; using more or less may make your symptoms worse.
If you use more Budesonide Nasal Spray than you should, continue with your usual dose. You are unlikely to experience any medical problems.
However if you have been using more than 4 sprays a day for more than a month consult your doctor immediately.
If you forget to take Budesonide Nasal Spray
If you forget to use your medicine on time, use it as soon as possible, then go back to your regular dosing schedule. Never use more sprays in one day than your regular dosing schedule, to make up for a missed dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
(angioedema): in very rare cases also severe allergic reaction is possible: if this happens you should contact your doctor immediately
• hives (an itchy rash that looks like nettle rash)
• rash
• itching
• irritation of the skin
Rare side effects (may affect up to 1 in 1,000 people):
These may occur after using this medicine for a long time:
• fragile bones
• increased pressure in the eyes
• clouding of the eye lenses
• slowing of the growth rate in children and adolescents, particularly after taking high doses for a long time
• adrenal supression. This may cause symptoms such as anorexia, abdominal pain, weight loss, nausea, headache, vomiting, decreased level of consciousness, low blood sugar levels and seizures. Situations which may potentially trigger acute adrenal crisis include trauma, infection, surgery or any rapid reduction in dosage. If you notice these symptoms you should contact your doctor immediately.
Very rare side effects (may affect up to 1 in 10,000 people), or where the frequency is not known (cannot be estimated from the available data):
• a hole in the cartilage dividing your nostrils
• voice disorder
• sore patches in your nose
This medicine can cause side effects on the whole body, particularly if high doses are used for a long time. These side effects are generally rare.
Potassium sorbate, an ingredient of this medicine, might cause irritation of the skin or mucous membranes, such as the inside if your nose.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C.
Do not freeze.
Discard the opened bottle with any remaining solution after 3 months.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6 Contents of the pack and other information
Like all medicines, this medicine can cause side effects, although not everybody gets them.
This medicine generally only treats symptoms affecting the nose (e.g. congestion or a “runny nose”). If you were previously treated with steroid tablets or injections but your doctor has now prescribed this medicine instead, you might experience a worsening of some of your other symptoms (e.g. red and itchy eyes). If this happens, your doctor will need to treat these other symptoms separately.
Side effects of nasal corticosteroids are more likely to occur if you have been using them at high doses for a number of months.
The following side effects can occur during treatment with Budesonide Nasal Spray:
Common side effects (may affect up to 1 in 10 people):
These may occur immediately after using this medicine:
• occasional sneezing, dry nose or stinging in your nose
• slightly bloody discharge from your nose
• nose bleed
Uncommon side effects (may affect up to 1 in 100 people):
• swollen face, tongue and/or pharynx and/or difficulty to swallow or hives together with difficulties to breathe
What Budesonide Nasal Spray contains
• The active substance is budesonide Each 0.05 ml dose (one spray) of this nasal spray, suspension, contains
64 micrograms of budesonide.
• The other ingredients are:
Dispersible cellulose (microcrystalline cellulose and carboxymethylcellulose sodium, (89:11, w/w))
Polysorbate 80
Potassium sorbate (E202)
Glucose, anhydrous Disodium edetate Hydrochloric acid, concentrated Ascorbic acid (E300)
Purified water
What Budesonide Nasal Spray Suspension looks like and contents of the pack
Budesonide Nasal Spray looks like a white homogeneous suspension.
Budesonide Nasal Spray is supplied as an amber type glass bottle fitted with a plastic nasal spray pump and polypropylene nasal applicator: pack size of 1 x 120 (1 x 10 ml) doses, 3 x 120 (3 x 10 ml) doses, 10x120 (10 x10 ml) doses.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Sandoz Ltd,
Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, UK.
Manufacturer:
Lek Pharmaceuticals d.d.,
Verovskova 57, 1526 Ljubljana,
Slovenia or
Salutas Pharma GmbH, Otto-von-Guericke-Allee 1,
39179 Barleben, Germany or
LEK S.A.,
ul. Podlipie 16,
95010 Strykow, Poland or LEK S.A.,
ul. Domaniewska 50C,
02-672 Warsaw, Poland.
This leaflet was last revised in 05/2014.
00000000
SZ00000LT000
|
Artwork Proof Box Ref: V013 - Art. 45 Paediatric WS (NL/W/0001/pdWS/001) | ||
|
Proof no. |
Date prepared: |
Font size: |
|
004.0 |
16/05/2014 |
8pt |
|
Colours: |
Fonts: | |
|
■ Black |
□ |
Helvetica |
|
□ |
□ | |
|
Dimensions: |
130 x 540 mm |
_y |