Enstilar 50 Micrograms/G + 0.5 Mg/G Cutaneous Foam
Package leaflet: Information for the patient
Enstilar® 50 micrograms/g + 0.5 mg/g cutaneous foam
calcipotriol/betamethasone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Enstilar® is and what it is used for
2. What you need to know before you use Enstilar®
3. How to use Enstilar®
4. Possible side effects
5. How to store Enstilar®
6. Contents of the pack and other information
1. What Enstilar® is and what it is used for
Enstilar is used on the skin to treat psoriasis vulgaris in adults. Psoriasis is caused by your skin cells being produced too quickly. This causes redness, scaling and thickness of your skin.
Enstilar contains calcipotriol and betamethasone.
Calcipotriol helps to bring the rate of skin cell growth back to normal and betamethasone helps to reduce the inflammation.
2. What you need to know before you use Enstilar®
Do not use Enstilar:
• if you are allergic to calcipotriol, betamethasone or any of the other ingredients of this medicine (listed in section 6)
• if you have problems with calcium levels in your blood (ask your doctor)
• if you have certain types of psoriasis called: erythrodermic psoriasis or pustular psoriasis (ask your doctor if you are unsure).
As Enstilar contains a strong steroid, do NOT use Enstilar on skin areas affected by:
• skin infections caused by viruses (e.g. cold sores or chicken pox)
• skin infections caused by a fungus (e.g. athlete's foot and ringworm)
• skin infections caused by bacteria
• skin infections caused by parasites (e.g. scabies)
• tuberculosis (TB)
• perioral dermatitis (red rash around the mouth)
• thin skin, easily damaged veins, stretch marks
• ichthyosis (dry skin with fish-like scales)
• acne (pimples)
• rosacea (severe flushing or redness of the skin on the face)
• ulcers and wounds.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Enstilar if:
• you have diabetes mellitus (diabetes), as your blood sugar level may be affected by the steroid
• you are using other medicines that contain steroids, as you may get side effects
• you have a certain type of psoriasis called guttate psoriasis.
Talk to your doctor, pharmacist or nurse during treatment if:
• you have used this medicine for a long period of time and plan to stop (as there is a risk your psoriasis will get worse or 'flare up' when steroids are stopped suddenly)
• your skin becomes infected, as you may need to stop your treatment
• the calcium level in your blood changes (see section 4 for further information).
Special precautions:
• avoid use under bandages or dressings as this increases the absorption of the steroid
• avoid use of more than 15 grams per day. This means that one 60 g can of Enstilar should last for at least 4 days.
15 g is dispensed if you fully depress the actuator for approximately 1 minute. Spraying for 2 seconds provides approximately 0.5 g of Enstilar. As a guide, 0.5 g of foam should cover an area of skin roughly corresponding to the surface area of an adult hand
• avoid use on more than 30% of your body
• avoid use on large areas of damaged skin, on mucous membranes or in skin folds (groin, armpits, under breasts) as this increases the absorption of the steroid
• avoid use on the face or on genitals (sexual organs) as they are very sensitive to steroids
• avoid excessive sunbathing, excessive use of a solarium and other forms of light treatment, as your skin is sensitive to light.
Children and adolescents
Enstilar is not recommended for the use in children below the age of 18 years.
Other medicines and Enstilar
Tell your doctor, pharmacist or nurse if you are using, have recently used, or might use any other medicines.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
If your doctor has agreed that you can breast-feed, take care and do not apply Enstilarto the breast area.
See 'Instruction for proper use'.
Driving and using machines
This medicine should not have any effect on your ability to drive or use machines.
Enstilar contains butylhydroxytoluene (E321)
This may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes.
See 'Instruction for proper use'.
3. How to use Enstilar®
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Enstilar is for cutaneous use (on the skin).
Before use, read the patient information, even if you have used Enstilar before.
Enstilar is designed for direct application (spray-on) to your skin affected by psoriasis vulgaris.
Instruction for proper use
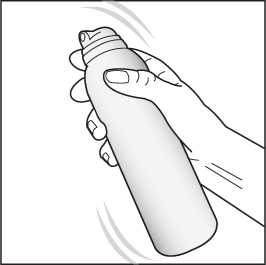
1. Shake the can for a few seconds before use.
2. Apply the foam by holding the can at least 3 cm from the skin and spray directly onto each affected area.
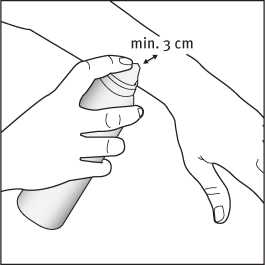
3. The foam can be sprayed holding the can in any orientation except horizontally.
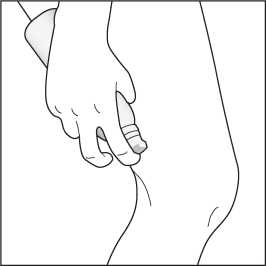
4. Gently rub the foam into each affected skin area.
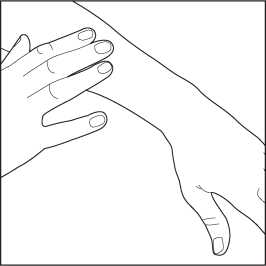
5. After applying the foam, put the cap back on the can to prevent accidental spraying when not in use.
6. Wash your hands well after using Enstilar (unless you are using the foam to treat your hands). This will avoid accidentally spreading the foam to other parts of your body (especially the face, mouth and eyes).
Further information for proper use:
• use only on your psoriasis and do not use on skin which does not have psoriasis
• wash or rinse well if, by accident, you have applied foam to your eyes, mouth, sexual organs or breasts if you are breast-feeding
• do not worry if some foam accidentally gets on normal skin near your psoriasis, but wipe it off if it spreads too far
• do not bandage, tightly cover or wrap the treated skin area
• in order to achieve optimal effect, it is recommended not to take a shower or bath immediately after spraying the foam
• after applying the foam, avoid contact with textiles which are easily stained by grease (e.g. silk).
Duration of treatment
• apply the foam once daily. It may be more convenient to use the foam in the evening.
• the normal treatment period is 4 weeks but your doctor may decide on a different treatment period.
If you have used more Enstilar than you should Important: One 60 g can of Enstilar should last for at least 4 days (see section 2 ’Special precautions’). If you use other calcipotriol containing medicines, the total amount of calcipotriol medicines, including Enstilar, must not exceed 15 grams per day. Contact your doctor if you have used more than the recommended dose.
Excessive use of Enstilar may cause a problem with calcium in your blood, which usually returns to normal when treatment is discontinued.
Excessive prolonged use can also cause your adrenal glands to stop working properly (the adrenal glands are found near the kidneys and produce hormones).
See section 4 for further information.
fXJ
fXJ
in
o
in
o
If you forget to use Enstilar
Do not use a double dose to make up for forgotten doses.
If you stop using Enstilar
The use of Enstilar should be stopped as indicated by your doctor. It may be necessary for you to stop this medicine gradually, especially if you have used it for a long time.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects that have been observed for Enstilar:
Uncommon side effects (may affect up to 1 in 100 people):
• allergic reaction. Symptoms may include skin rash and swelling of the skin, face or mouth
• worsening or 'flare up' of your psoriasis after treatment has stopped
• a rise in calcium levels in the blood
• skin irritation which might include itching, burning and stinging and/or redness of the skin
• redness, pain or swelling of the hair roots of the skin (folliculitis)
• loss of skin colour (depigmentation).
Enstilar contains betamethasone (a strong steroid) and calcipotriol. You may therefore experience the following side effects. These side effects are more likely to happen if Enstilar is used for a long time, if used under dressings or in skin folds (e.g. groin, armpits or under breasts), or if used on large skin areas:
• allergic reactions with swelling of the face or other parts of the body such as the hands or feet. Swelling of the mouth/throat and trouble breathing may also occur
• calcium levels in your blood or urine may increase so much that you get symptoms. Signs are frequent urination, constipation, muscle weakness, and confusion. When the treatment is stopped, the calcium levels return to normal
• your adrenal glands may stop working properly. Signs are tiredness, depression, anxiety
• cloudy vision, difficulty seeing at night, sensitivity to light (this could be a sign of cataracts)
• eye pain, red eye, decreased or cloudy vision (this could be a sign of increased pressure inside the eye)
• infections (because your immune system is weakened)
• pustular psoriasis (a red area of psoriasis with yellowish pustules (pimples))
• you may experience fluctuations in blood sugar levels.
If you experience any of the above side effects you should contact your doctor immediately.
Less serious side effects known to be caused by calcipotriol or betamethasone include the following:
• thinning of the skin
• stretch marks
• blood vessels under your skin may become more noticable
• changes in hair growth
• red rash around the mouth (perioral dermatitis)
• worsening of your psoriasis
• sensitivity of the skin to light resulting in a rash
• itchy skin rash (eczema)
• white or grey hair can transiently change to a yellowish colour at the application site when used on the scalp.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system.
For Ireland, via HPRA Pharmacovigilance,
Earlsfort Terrace, IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie E-mail: medsafety@hpra.ie
For the United Kingdom, via the Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Enstilar®
Caution: Extremely flammable aerosol. Pressurised container: May burst if heated. Protect from sunlight. Do not expose to temperatures exceeding 50°C. Do not pierce or burn, even after use. Do not spray on an open flame or other ignition source. Keep away from sparks, open flames and other ignition sources. No smoking near the can.
Keep this medicine out of the sight and reach of children.
Do not use Enstilar after the expiry date, which is stated on the carton and can after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C.
The can should be discarded 6 months after first opening. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6.Contents of the pack and other information What Enstilar contains
The active substances are:
Calcipotriol and betamethasone.
One gram of cutaneous foam contains 50 micrograms of calcipotriol (as monohydrate) and 0.5 mg of betamethasone (as dipropionate).
The excipients are:
Liquid paraffin
Polyoxypropylene stearyl ether All-rac-a-tocopherol White soft paraffin Butylhydroxytoluene (E321)
Butane
Dimethyl ether.
What Enstilar looks like and contents of the pack
Enstilar is a cutaneous foam.
After spraying, a white to off-white foam is formed. Aluminium can with a polyamide-imide inner lacquer, equipped with a continuous valve and actuator.
The can contains 60 g of foam, not including the amount of propellants.
Pack sizes: 60 g, 2 x 60 g.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder
LEO Pharma A/S Industriparken 55 DK-2750 Ballerup Denmark
Manufacturer
Colep Laupheim GmbH & Co. KG FockestraGe 12 DE-88471 Laupheim Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
Enstilar: Austria, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Portugal, Slovak Republic, Spain, Sweden, United Kingdom Enstilum: Belgium, Luxembourg, Romania, Slovenia
This leaflet was last revised in March 2016.
Detailed information on this medicine is available on the website of the Health Products Regulatory Authority, www.hpra.ie. and Medicines and Healthcare products Regulatory Agency, www.mhra.gov.uk.
LEO 050522