Enstilar 50 Micrograms/G + 0.5 Mg/G Cutaneous Foam
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Enstilar 50 micrograms/g + 0.5 mg/g cutaneous foam
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
One gram of cutaneous foam contains 50 micrograms of calcipotriol (as monohydrate) and 0.5 mg of betamethasone (as dipropionate).
Excipient with known effect:
Butylhydroxytoluene (E321) 50 micrograms/g cutaneous foam.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Cutaneous foam.
After spraying, a white to off-white foam is formed.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Topical treatment of psoriasis vulgaris in adults.
4.2 Posology and method of administration
Posology
Enstilar foam should be applied to the affected area once daily. The recommended treatment period is 4 weeks. The daily maximum dose of Enstilar should not exceed 15 g, i.e. one 60g can should last for at least 4 days. 15 g corresponds to the amount administered from the can if the actuator is fully depressed for approximately one minute. A two-second application delivers approximately 0.5 g. As a guide, 0.5 g of foam should cover an area of skin roughly corresponding to the surface area of an adult hand.
If using other topical products containing calcipotriol in addition to Enstilar, the total dose of all calcipotriol containing products should not exceed 15 g per day.
The total body surface area treated should not exceed 30%.
Special populations
Renal and hepatic impairment
The safety and efficacy of Enstilar foam in patients with severe renal insufficiency or severe hepatic disorders have not been evaluated.
Paediatric population
The safety and efficacy of Enstilar foam in children below 18 years have not been established. No data are available.
Method of administration
For cutaneous use.
The can should be shaken for a few seconds before use. Enstilar should be applied by spraying holding the can at least 3 cm from the skin. The foam can be sprayed holding the can in any orientation except horizontally.
Enstilar should be sprayed directly onto each affected skin area and rubbed in gently. The hands should be washed after using Enstilar (unless Enstilar is used to treat the hands) to avoid accidentally spreading to other parts of the body. Application under occlusive dressings should be avoided since it increases the systemic absorption of corticosteroids. It is recommended not to take a shower or bath immediately after application of Enstilar.
4.3 Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Enstilar is contraindicated in erythrodermic and pustular psoriasis.
Due to the content of calcipotriol, Enstilar is contraindicated in patients with known disorders of calcium metabolism (see section 4.4).
Due to the content of corticosteroid, Enstilar is contraindicated in the following conditions if present in the treatment area: viral (e.g. herpes or varicella) lesions of the skin, fungal or bacterial skin infections, parasitic infections, skin manifestations in relation to tuberculosis, perioral dermatitis, atrophic skin, striae atrophicae, fragility of skin veins, ichthyosis, acne vulgaris, acne rosacea, rosacea, ulcers, and wounds (see section4.4).
4.4 Special warnings and precautions for use
Effects on endocrine system:
Adverse reactions found in connection with systemic corticosteroid treatment, such as adrenocortical suppression or impaired glycaemic control of diabetes mellitus may occur also during topical corticosteroid treatment due to systemic absorption.
Application under occlusive dressings should be avoided since it increases the systemic absorption of corticosteroids. Application on large areas of damaged skin, or on mucous membranes or in skin folds should be avoided since it increases the systemic absorption of corticosteroids (see section 4.8).
Effects on calcium metabolism:
Due to the content of calcipotriol in Enstilar, hypercalcaemia may occur. Serum calcium is normalised when treatment is discontinued. The risk of hypercalcaemia is minimal when the maximum daily dose of Enstilar (15 g) is not exceeded (see section 4.2).
Local adverse reactions:
Enstilar contains a potent group III-steroid and concurrent treatment with other steroids on the same treatment area must be avoided.
Skin of the face and genitals are very sensitive to corticosteroids. The medicinal product should not be used in these areas.
The patient must be instructed in correct use of the product to avoid application and accidental transfer to the face, mouth, and eyes. Hands must be washed after each application to avoid accidental transfer to these areas.
Concomitant, skin infections:
When lesions become secondarily infected, they should be treated with antimicrobiological therapy. However, if infection worsens, treatment with corticosteroids should be discontinued (see section 4.3).
Discontinuation of treatment:
When treating psoriasis with topical corticosteroids, there may be a risk of rebound effects when discontinuing treatment. Medical supervision should therefore continue in the post-treatment period.
Long-term use:
Long-term use of corticosteroids may increase the risk of local and systemic adverse reactions. Treatment should be discontinued in case of adverse reactions related to long-term use of corticosteroid (see section 4.8).
Unevaluated use:
There is no experience with the use of Enstilar in guttate psoriasis.
UV exposure:
During Enstilar treatment, physicians are recommended to advise patients to limit or avoid excessive exposure to either natural or artificial sunlight. Topical calcipotriol should be used with UVR only if the physician and patient consider that the potential benefits outweigh the potential risks (see section 5.3).
Adverse reactions to excipients:
Enstilar contains butylhydroxytoluene (E321) as an excipient, which may cause local skin reactions (e.g. contact dermatitis) or irritation to the eyes and mucous membranes.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with Enstilar.
4.6 Fertility, pregnancy and lactation
Pregnancy:
There are no adequate data from the use of Enstilar in pregnant women. When administered orally in animals, studies of calcipotriol have not shown teratogenic effects, though reproductive toxicity has been shown (see section 5.3). Studies in animals with glucocorticoids have shown reproductive toxicity (see section 5.3), but a number of epidemiological studies (less than 300 pregnancy outcomes) have not revealed congenital anomalies among infants born to women treated with corticosteroids during pregnancy. The potential risk for humans is uncertain. Therefore, during pregnancy, Enstilar should only be used when the potential benefit justifies the potential risk.
Breast-feeding:
Betamethasone passes into breast milk, but risk of an adverse reaction in the infant seems unlikely with therapeutic doses. There are no data on the excretion of calcipotriol in breast milk. Caution should be exercised when prescribing Enstilar to women who breast-feed. The patient should be instructed not to use Enstilar on the breast when breast-feeding.
Fertility:
Studies in rats with oral doses of calcipotriol or betamethasone dipropionate demonstrated no impairment of male and female fertility (see section 5.3).
4.7 Effects on ability to drive and use machines
Enstilar has no or negligible influence on the ability to drive and to use machines.
4.8 Undesirable effects
The estimation of the frequency of adverse reactions is based on a pooled analysis of data from clinical studies.
The most frequently reported adverse reactions during treatment are application site reactions.
Adverse reactions are listed by MedDRA SOC and the individual adverse reactions are listed starting with the most frequently reported. Within each frequency grouping, adverse reactions are presented in the order of decreasing seriousness.
Very common (>1/10)
Common (>1/100 to <1/10)
Uncommon (>1/1,000 to <1/100)
Rare (>1/10,000 to <1/1,000)
Very rare (<1/10,000)
Not known (cannot be estimated from the available data)
|
Infections and infestations | |
|
Uncommon >1/1,000 to <1/100 |
Folliculitis |
|
Immune system disorders | |
|
Uncommon >1/1,000 to <1/100 |
Hypersensitivity |
|
Metabolism and nutrition disorders | |
|
Uncommon >1/1,000 to <1/100 |
Hypercalcaemia* |
|
Skin and subcutaneous tissue disorders | |
|
Uncommon >1/1,000 to <1/100 |
Skin hypopigmentation |
|
Not known |
Haircolour changes** |
|
General disorders and administration site conditions | |
|
Uncommon >1/1,000 to <1/100 |
Rebound effect Application site pruritus Application site irritation |
*Mild hypercalcaemia has been observed.
**Transient discolouration of the hair at scalp application site, to a yellowish colour in white or grey hair, has been reported for calcipotriol and betamethasone combination products.
The following adverse reactions are considered to be related to the pharmacological classes of calcipotriol and betamethasone, respectively:
Calcipotriol:
Adverse reactions include application site reactions, pruritus, skin irritation, burning and stinging sensation, dry skin, erythema, rash, dermatitis, psoriasis aggravated, photosensitivity and hypersensitivity reactions, including very rare cases of angioedema and facial oedema.
Systemic effects after topical use may appear very rarely causing hypercalcaemia or hypercalciuria (see section 4.4).
Betamethasone (as dipropionate):
Local reactions can occur after topical use, especially during prolonged application, including skin atrophy, telangiectasia, striae, folliculitis, hypertrichosis, perioral dermatitis, allergic contact dermatitis, depigmentation, and colloid milia.
When treating psoriasis with topical corticosteroids, there may be a risk of generalised pustular psoriasis.
Systemic reactions due to topical use of corticosteroids are rare in adults; however, they can be severe. Adrenocortical suppression, cataract, infections, impaired glycaemic control of diabetes mellitus, and increase of intra-ocular pressure can occur, especially after long-term treatment. Systemic reactions occur more frequently when applied under occlusion (plastic, skin folds), when applied on large areas, and during long-term treatment (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Usage above the recommended dose may cause elevated serum calcium which subsides when treatment is discontinued. The symptoms of hypercalcaemia include polyuria, constipation, muscle weakness, confusion, and coma.
Excessive prolonged use of topical corticosteroids may result in adrenocortical suppression which is usually reversible. Symptomatic treatment may be indicated.
In case of chronic toxicity the corticosteroid treatment must be discontinued gradually.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antipsoriatics. Other antipsoriatics for topical use, Calcipotriol, combinations. ATC Code: D05AX52.
Mechanism of action:
Enstilar foam combines the pharmacological effects of calcipotriol hydrate as a synthetic vitamin D3 analogue and betamethasone dipropionate as a synthetic corticosteroid.
In psoriasis, vitamin D and its analogues act mainly to inhibit keratinocyte proliferation and induce keratinocyte differentiation. The underlying antiproliferative mechanism of vitamin D in keratinocytes involves the induction of the growth inhibitory factor transforming growth factor^ and of cyclin-dependent kinase inhibitors, with subsequent growth arrest in the G1 phase of the cell cycle plus down-regulation of the two proliferation factors early growth response-1 and polo-like kinase-2.
In addition, vitamin D has an immunomodulatory effect, suppressing activation and differentiation of Th17/Th1 cells while inducing a Th2/Treg response.
In psoriasis, corticosteroids suppress the immune system, particularly pro-inflammatory cytokines and chemokines, thereby inhibiting T-cell activation. At the molecular level, corticosteroids act via the intracellular glucocorticoid receptor and the anti-inflammatory function is due to transrepression of pro-inflammatory transcription factors such as nuclear factor kB, activator protein-1, and interferon regulatory factor-3.
In combination, calcipotriol monohydrate and betamethasone dipropionate promote greater anti-inflammatory and anti-proliferative effects than either component alone.
Pharmacodynamic effects:
Under maximum use conditions, in subjects with extensive psoriasis on the body and scalp treated for up to 4 weeks, adrenal response to ACTH was determined by measuring serum cortisol levels. None of 35 subjects had suppressed serum cortisol levels at 30 or 60 minutes post ACTH stimulation. Thus it appears that for Enstilar, the risk of adrenal suppression is low when applied to extensive psoriasis vulgaris for 4 weeks. Similarly, there was no indication of abnormal calcium metabolism following application of Enstilar to extensive psoriasis vulgaris for 4 weeks.
Clinical efficacy:
The efficacy of once daily use of Enstilar has been investigated in three randomised, double-blind or investigator-blind, 4-week clinical trials including more than 1,100 subjects with psoriasis on the body (also scalp in Trial Two) of at least mild severity according to the Physician’s Global Assessment of disease severity (PGA), affecting at least 2% body surface area (BSA), and with a modified Psoriasis Area Severity Index (m-PASI) of at least 2. The physician’s global assessment is made using a 5-point scale (clear, almost clear, mild, moderate, and severe) based on the average psoriatic lesion. The m-PASI is a composite score assessing severity (erythema, scale, and induration) and affected area (excluding face and skin folds).
The number of subjects in each of the three trials and the number of subjects randomised to each treatment group are included in the tables below.
The primary endpoint was subjects with ‘treatment success’ (‘clear’ or ‘almost clear’ for subjects with at least moderate disease at baseline, ‘clear’ for subjects with mild disease at baseline) according to the PGA at Week 4.
Disease-related baseline characteristics
|
Trial One (N=426) |
Trial Two (N=302) |
Trial Three (N=376) | |
|
Baseline disease severity (PGA): Mild Moderate Severe |
65 (15.3%) 319 (74.9%) 42 (9.9%) |
41 (13.6%) 230 (76.2%) 31 (10.3%) |
63 (16.8%) 292 (77.7%) 21 (5.6%) |
|
Mean BSA (range) |
7.5% (2-30%) |
7.1% (2-28%) |
7.5% (230%) |
|
Mean m-PASI (range) |
7.5 (2.0-47.0) |
7.6 (2.0-28.0) |
6.8 (2.0-22.6) |
Percentage of subjects with ‘treatment success’ according to the PGA of the body at Week 4
|
Enstilar |
Foam vehicle |
BDP in foam vehicle |
Calcipotriol in foam vehicle |
Daivobet Ointment |
Ointment vehicle | |
|
Trial One |
(N=323) 53.3% |
(N=103) 4.8% |
- |
- |
- |
- |
|
Trial Two |
(N=100) 45.0% |
- |
(N=101) 30.7% |
(N=101) 14.9% |
- |
- |
|
Trial Three |
(N=141) 54.6% |
(N=49) 6.1% |
- |
- |
(N=135) 43.0% |
(N=51) 7.8% |
Results for the primary endpoint ‘treatment success’ (PGA) of body at Week 4 showed Enstilar to be statistically significantly more effective than all the comparators included and responses were observed in all categories of baseline disease severity.
In Trial Two, the effect of Enstilar on scalp psoriasis was investigated as the percentage of subjects with ‘treatment success’ according to the PGA of the scalp at Week 4.
Percentage of subjects with ‘treatment success’ according to the PGA of the scalp at Week 4
|
Enstilar |
BDP in foam |
Calcipotriol in foam | |
|
vehicle |
vehicle | ||
|
Trial Two |
(N=100) |
(N=101) |
(N=101) |
|
53.0 % |
47.5 % |
35.6 % |
Enstilar was statistically significantly more effective compared to calcipotriol and also associated with a higher rate of treatment success than BDP but this comparison did not reach statistical significance.
The effect of Enstilar on itch and itch-related sleep loss was investigated in Trial One using a visual analogue scale (VAS) ranging from 0 mm (no itch/no sleep loss at all) to 100 mm (worst itch you can imagine/worst possible sleep loss). A statistically significantly higher number of subjects in the Enstilar group compared to vehicle achieved a 70% reduction in itch and itch-related sleep loss from Day 3 and throughout the treatment period.
Percentage of subjects achieving at least 70% reduction in itch compared to baseline in Trial One (for subjects who reported itch at baseline)
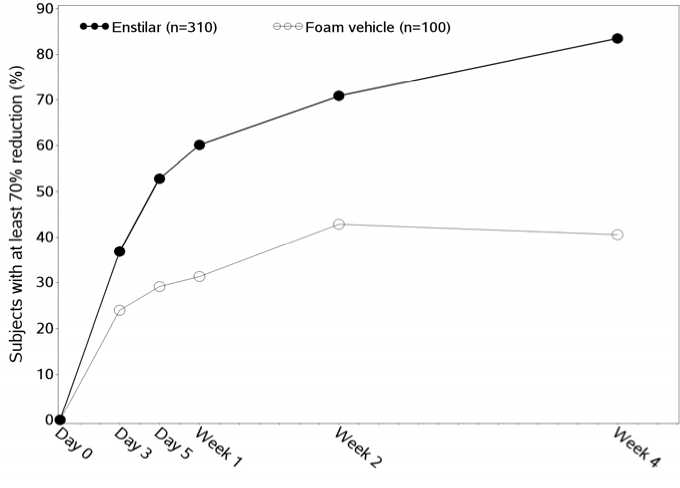
The effect of Enstilar on quality of life was investigated in Trial One using the generic EQ-5D-5L questionnaire and the dermatologically specific DLQI questionnaire. Statistically significantly greater improvement in quality of life in favour of Enstilar was demonstrated for DLQI from Week 1 and throughout the treatment period and for EQ-5D-5L at Week 4.
5.2 Pharmacokinetic properties
Following systemic exposure, both active ingredients - calcipotriol and betamethasone dipropionate - are rapidly and extensively metabolised.
The main route of excretion of calcipotriol is via faeces (rats and minipigs) and for betamethasone dipropionate it is via urine (rats and mice). In rats, tissue distribution studies with radiolabelled calcipotriol and betamethasone dipropionate showed that the kidney and liver had the highest level of radioactivity.
The extent of percutaneous absorption of the two active ingredients following topical application of Enstilar was determined in the HPA axis trial in subjects with extensive psoriasis vulgaris (see section 5.1). Calcipotriol and betamethasone dipropionate were below the lower limit of quantification in most samples from 35 patients treated once daily for 4 weeks for extensive psoriasis involving the body and scalp. Calcipotriol was quantifiable at some time point in 1 subject, betamethasone dipropionate in 5 subjects and metabolites of calcipotriol and betamethasone dipropionate were detectable in 3 and 27 subjects, respectively.
5.3 Preclinical safety data
Studies of corticosteroids in animals have shown reproductive toxicity (cleft palate, skeletal malformations). In reproduction toxicity studies with long-term oral administration of corticosteroids to rats, prolonged gestation and prolonged and difficult labour were detected. Moreover, reduction in offspring survival, body weight and body weight gain was observed. There was no impairment of fertility. The relevance for humans is unknown.
Calcipotriol has shown maternal and foetal toxicity in rats and rabbits when given by the oral route at doses of 54 pg/kg/day and 12 pg/kg/day, respectively. The foetal abnormalities observed with concomitant maternal toxicity included signs indicative of skeletal immaturity (incomplete ossification of the pubic bones and forelimb phalanges, and enlarged fontanelles) and an increased incidence of supernumerary ribs.
The estimated systemic exposure following topical application of Enstilar to psoriasis patients is negligible compared to the concentrations of calcipotriol evaluated in the oral in vivo studies, and there is no appreciable reproductive risk to humans receiving therapeutic exposure to Enstilar.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity and genotoxicity.
A dermal carcinogenicity study with calcipotriol in mice and an oral carcinogenicity study in rats revealed no special risk to humans.
Photo(co)carcinogenicity studies in mice suggest that calcipotriol may enhance the effect of UVR to induce skin tumours.
A dermal carcinogenicity study in mice and an oral carcinogenicity study in rats revealed no special risk of betamethasone dipropionate to humans.
In a local tolerability study in minipigs, Enstilar caused mild to moderate skin irritation.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Liquid paraffin
Polyoxypropylene stearyl ether All-rac-a-tocopherol White soft paraffin Butylhydroxytoluene (E321)
Butane
Dimethyl ether
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years.
After first opening: 6 months.
6.4 Special precautions for storage
Do not store above 30°C.
Caution:
Extremely flammable aerosol.
Pressurised container: May burst if heated.
Protect from sunlight.
Do not expose to temperatures exceeding 50°C.
Do not pierce or burn, even after use.
Do not spray on an open flame or other ignition source.
Keep away from sparks, open flames and other ignition sources. No smoking.
6.5 Nature and contents of container
Aluminium can with a polyamide-imide inner lacquer, equipped with a continuous valve and actuator.
The can contains 60 g of foam, not including the amount of propellants.
Pack sizes: 60 g and 2 x 60 g Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
LEO Pharma A/S Industriparken 55 DK-2750 Ballerup Denmark
8 MARKETING AUTHORISATION NUMBER(S)
PL 05293/0008
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
15/04/2016
10 DATE OF REVISION OF THE TEXT
15/04/2016