Erastig 4.6 Mg/24 H Transdermal Patch
2
|
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16
TEVA UK Ref: 231-30-57007-E LEA RIVASTIGMINE TRANSD PATCH 4.6-9.5MG/24HR 30 TAB TUK<SK
Version: 3
02 July 2015
|
o | ||
Erastig might interfere with anticholinergic m< relieve stomach cramps or spasms (e.g. dicyclc amantadine) or to prevent motion sickness (e.g meclizine)
edicines some of which are medicines used to mine), to treat Parkinson's disease (e.g.
. diphenhydramine, scopolamine, or
Package leaflet:
Information for the user
Erastig 4.6 mg/24 h
transdermal patch
Erastig 9.5 mg/24 h
transdermal patch
Rivastigmine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you, only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
WHAT IS IN THIS LEAFLET:
1. What Erastig is and what it is used for
2. What you need to know before you use Erastig
3. How to use Erastig
4. Possible side effects
5. How to store Erastig
6. Contents of the pack and other information
WHAT ERASTIG IS AND WHAT IT IS USED FOR
The active substance of Erastig is rivastigmine.
Rivastigmine belongs to a class of substances called cholinesterase inhibitors. In patients with Alzheimer's dementia, certain nerve cells die in the brain, resulting in low levels of the neurotransmitter acetylcholine (a substance that allows nerve cells to communicate with each other). Rivastigmine works by blocking the enzymes that break down acetylcholine: acetylcholinesterase and butyrylcholinesterase. By blocking these enzymes, Erastig allows levels of acetylcholine to be increased in the brain, helping to reduce the symptoms of Alzheimer's disease.
Erastig is used for the treatment of adult patients with mild to moderately severe Alzheimer's dementia, a progressive brain disorder that gradually affects memory, intellectual ability and behaviour.
WHAT YOU NEED TO KNOW BEFORE YOU USE ERASTIG
Do not use Erastig
- if you are allergic to rivastigmine (the active substance in Erastig ) or any of the other ingredients of this medicine (listed in section 6)
- if you have ever had an allergic reaction to a similar type of medicine (carbamate derivatives).
- if you have a skin reaction spreading beyond the patch size, if there is a more intense local reaction (such as blisters, increasing skin inflammation, swelling) and if it does not improve within 48 hours after removal of the transdermal patch.
If this applies to you, tell your doctor and do not apply Erastig transdermal patches.
Talk to your doctor, pharmacist or nurse before using Erastig
- if you have, or have ever had, an irregular heartbeat.
- if you have, or have ever had, an active stomach ulcer.
- if you have, or have ever had, difficulties in passing urine.
- if you have, or have ever had, seizures.
- if you have, or have ever had, asthma or a severe respiratory disease.
- if you suffer from trembling.
- if you have a low body weight.
- if you have gastrointestinal reactions such as feeling sick (nausea), being sick (vomiting) and diarrhoea. You may become dehydrated (losing too much fluid) if vomiting or diarrhoea are prolonged.
- if you have impaired liver function.
If any of these apply to you, your doctor may need to monitor you more closely while you are on this medicine.
If you have not applied a Erastig patch for several days, do not apply the next one before you have talked to your doctor.
There is no relevant use of Erastig in the paediatric population in the treatment of Alzheimer's disease.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
If you have to undergo surgery whilst using Era that you are using them because they may exa< during anaesthesia.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you baby, ask your doctor or pharmacist for advice
stig transdermal patches, tell your doctor ggerate the effects of some muscle relaxants
may be pregnant or are planning to have a before taking this medicine.
g transdermal patches must be assessed d. Erastig should not be used during
If you are pregnant, the benefits of using Erast against the possible effects on your unborn chil pregnancy unless clearly necessary.
You should not breast-feed during treatment with Erastig transdermal patches.
Your doctor will tell you whether your illness a safely. Erastig transdermal patches may cause confused do not drive, use machines or perfor
lows you to drive vehicles and use machines fainting or severe confusion. If you feel faint or m any other tasks that require your attention.
3.
HOW TO USE ERASTIG
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
IMPORTANT:
• Take off the previous patch before putting ONE new patch on.
• Only one patch per day.
• Do not cut the patch into pieces.
• Press the patch firmly in place for at least ■
During the course of the treatment your doctor may adjust the dose to suit your individual needs.
If you have not applied a patch for three days, do not apply the next one before you have talked to your doctor. Transdermal patch treatment can be resumed at the same dose if treatment is not interrupted for more than three days. Otherwise your doctor will restart your treatment on Erastig 4.6 mg/24 h.
Erastig can be used with food, drink and alcohol.
Where to apply your Erastig transdermal patch
• Before you apply a patch, make sure that your skin is:
- clean, dry and hairless
- free of any powder, oil, moisturiser or lotion that could keep the patch from sticking to your skin properly
- free of cuts, rashes and/or irritations.
• Carefully remove any existing patch before putting on a new one. Having multiple patches on your body could expose you to an excessive amount of this medicine which could be potentially dangerous.
• Apply only one patch per day to only one of the possible locations, as shown in the following diagrams:
- left upper arm or right upper arm
- left upper chest or right upper chest (avoid breast)
- left upper back or right upper back
- left lower back or right lower back
Every 24 hours take off the previous patch before putting ONE new patch on to ONLY ONE of the following possible locations.
How to apply your Erastig transdermal patch
Erastig patches are thin, translucent, plastic patches that stick to the skin. Each patch is sealed in a sachet that protects it until you are ready to put it on. Do not open the sachet or remove a patch until just before you apply it.
Carefully remove the existing patch before putting on a new one.
For patients starting treatment for the first time and for patients restarting "Erastig"after treatment interruption, please begin with the second picture.
- Each patch is sealed in its own protective sachet. You should only open the sachet when you are ready to apply the patch.
Cut the sachet along the dotted line with scissors and remove the patch from the sachet.
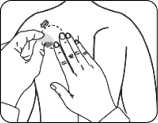

A protective liner covers the sticky side of the patch. Peel off one side of the protective liner and do not touch the sticky part of the patch with the fingers.
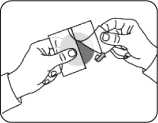
30 seconds using the palm of the hand.
Your doctor will tell you which Erastig transderi
• Treatment usually starts with Erastig 4.6 mg
• The recommended usual daily dose is Erastig physician may consider increasing the dose
• Only wear one Erastig patch at a time and re
mal patch is most suitable for you.
24 h.
9.5 mg/24 h. If well tolerated, the treating to 13.3 mg/24 h.
place the patch with a new one after 24 hours.
Front
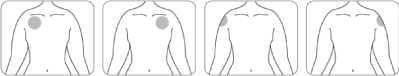
Or Or Or Or
Back
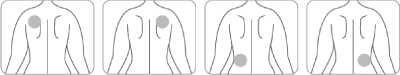
Or Or Or
When changing the patch, you must remove the previous day's patch before you apply the new one to a different location of skin each time (for example on the right side of your body one day, then on the left side the next day, and on your upper body one day, then on your lower body the next day). Do not apply a new patch to that same location of skin twice
within 14 days.
Put the sticky side of the patch on the upper or lower back, upper arm or chest and then peel off the second side of the protective liner.
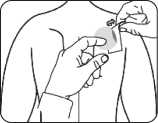
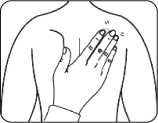
- Then press the patch firmly in place for at least 30 seconds using the palm of the hand to make sure that the edges stick well.
If it helps you, you may write, for example, the day of the week, on the patch with a thin ball point pen.
code area
from the available data) such as blisters or inflamed skin such as tremor, stiffness and shuffling serious upper stomach pain, often with
cinations)
whites of eyes, abnormal darkening of the ness and loss of appetite) iver is working
ht away, if you notice any of the side effects
sm.
code area
The patch should be worn continuously until it is time to replace it with a new one. You may wish to experiment with different locations when applying a new patch, to find ones that are most comfortable for you and where clothing will not rub on the patch.
How to remove your Erastig transdermal patch
Gently pull at one edge of the patch to remove it completely from the skin. In case the adhesive residue is left over on your skin, gently soak the area with warm water and mild soap or use baby oil to remove it. Alcohol or other dissolving liquids (nail polish remover or other solvents) should not be used.
You should wash your hands with soap and water after removing the patch. In case of contact with eyes or if the eyes become red after handling the patch, rinse immediately with plenty of water and seek medical advice if symptoms do not resolve.
Can you wear your Erastig transdermal patch when you are bathing, swimming, or in the sun?
Bathing, swimming or showering should not affect the patch. Make sure the patch does not loosen during these activities.
Do not expose the patch to any external heat sources (e.g. excessive sunlight, saunas, solarium) for long periods of time.
What to do if a patch falls off
If a patch falls off, apply a new one for the rest of the day, then replace it at the same time as usual the next day.
When and for how long to apply your Erastig transdermal patch
To benefit from treatment, you must apply a new patch every day, preferably at the same time of day.
Only wear one Erastig patch at a time and replace the patch with a new one after 24 hours. If you use more Erastig than you should
If you accidentally apply more than one patch, remove all the patches from your skin, then inform your doctor that you have accidentally applied more than one patch. You may require medical attention. Some people who have accidentally taken too much rivastigmine have experienced feeling sick (nausea), being sick (vomiting), diarrhoea, high blood pressure and hallucinations. Slow heart beat and fainting may also occur.
If you find you have forgotten to apply a patch, apply one immediately. You may apply the next patch at the usual time the next day. Do not apply two patches to make up for the one that you missed.
Tell your doctor or pharmacist if you stop using the patch.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You may have side effects more often when you start your medicine or when your dose is increased. Usually, the side effects will slowly go away as your body gets used to the medicine.
Take off your patch and tell your doctor straight away, if you notice any of the following side effects which could become serious:
Common (may affect up to 1 in 10 people)
Loss of appetite, decreased appetite Feeling dizzy Feeling agitated or sleepy Urinary tract infection
Urinary incontinence (inability to retain adequate urine)
Stomach problems such as feeling sick (nausea) or being sick (vomiting), diarrhoea, indigestion and stomach pain.
Anxiety Depression Headache Fainting
Feeling tired or weak Fever
Weight loss Delirium Rash
A skin reaction where the patch was used, such as redness, rash, swelling, inflammation or irritation of the skin.
Uncommon (may affect up to 1 in 100 people)
• Problems with your heartbeat such as slow heartbeat
• Stomach ulcer
• Dehydration (losing too much fluid)
• Hyperactivity (high level of activity, restlessness)
• Aggression.
Rare (may affect up to 1 in 1,000 people)
• Falling.
Very rare (may affect up to 1 in 10,000 peopl
• Stiff arms or legs
• Trembling hands.
Not known (frequency cannot be estimated
• Allergic reaction where the patch was used,
• The signs of Parkinson's disease get worse
• Inflammation of the pancreas-signs include feeling sick (nausea) or being sick (vomiting
• Fast or uneven heartbeat
• Seeing things that are not really there (hallu
• High blood pressure
• Fits (seizures)
• Liver disorders (yellow skin, yellowing of the urine or unexplained nausea, vomiting, tirec
• Changes in tests which show how well the
• Feeling restless.
Take off your patch and tell your doctor straig above.
Other side effects seen with capsules or oral solutions containing rivastigmine and which may occur with the patch:
Common (may affect up to 1 in 10 people)
• Too much saliva
• Generally feeling unwell
• Trembling or feeling confused
• Increased sweating.
Uncommon (may affect up to 1 in 100 peopl
• Difficulty sleeping.
Rare (may affect up to 1 in 1,000 people)
• Chest pain - this may be caused by heart spa
Very rare (may affect up to 1 in 10,000 people)
• Bleeding in the gut - shows as blood in stools or when being sick
• Some people who have been violently sick have had tearing of the tube that connects your mouth with your stomach (oesophagus).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ERASTIG
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the carton and sachet after {EXP}. The expiry date refers to the last day of the month.
Store in the original package in order to protect from light.
Keep the transdermal patch in the sachet until use.
This medicinal product does not require any special temperature storage conditions
Do not use any patch that is damaged or shows signs of tampering.
After removing a patch, fold it in half with the sticky sides on the inside and press them together. Return the used patch to its sachet and dispose of it in such a way that children cannot handle it. Do not touch your eyes with your fingers and wash your hands with soap and water after removing the patch. If your community burns domestic rubbish, you can dispose of the patch with your domestic rubbish. Otherwise, return used patches to a pharmacy, preferably in the original packaging.
6. CONTENT OF THE PACK AND OTHER INFORMATION
The active substance is rivastigmine.
[Erastig 4.6 mg/24 h transdermal patch:]
Each patch releases 4.6 mg rivastigmine per 24 hours is 5 cm2 and contains 9 mg rivastigmine [Erastig 9.5 mg/24 h transdermal patch:]
Each patch releases 9.5 mg rivastigmine per 24 hours is 10 cm2 and contains 18 mg rivastigmine.
Film: Polyester film
Fluoro-coated polyester film
Drug matrix: Acrylic adhesive, Acrylates copolymer
poly(butyl methacrylat-co-methyl methacrylat)
Adhesive matrix: Silicone adhesive Printing ink: Black printing ink
What Erastig looks like and contents of the pack
Each transdermal patch is a thin patch consisting of three layers. The outer layer is translucent, white and black-printed with the following:
[Erastig 4.6 mg/24 h transdermal patch:]
"Rivastigmine", "4.6 mg/24 h"
[Erastig 9.5 mg/24 h transdermal patch:]
"Rivastigmine", "9.5 mg/24 h"
One transdermal patch is packed in one child-resistant and heat-sealed sachet. The patches ar available in packs containing 7, 10, 30, 60 and 90 sachets and in multipacks containing 60 (2 x 30) and 90 (3 x 30) sachets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Teva UK Limited, Eastbourne, BN22 9AG, UK
Manufacturer
PHAST Gesellschaft fur Pharmazeutische Qualitatsstandards mbH Kardinal-Wendel-Strasse 16 66424 Homburg Germany
This leaflet was last revised in 07/2015
PL 00289/1814 57007-E
PL 00289/1815 231829.03-GB
70
oo