Latanoprost 0.005% Eye Drops
Out of date information, search another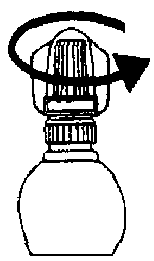
4.
5.
6. 7.
8.
9.
Assessed against UK PIL dated July 2009
PACKAGE LEAFLET: INFORMATION FOR THE USER
Xalatan® 0.005% Eye Drops Latanoprost 0.005% Eye Drops
_(latanoprost)_
This product is available using the names Xalatan 0.005% Eye Drops or Latanoprost 0.005% Eye Drops, but will be referred to as Xalatan throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine. Even if you have already used Xalatan or a similar medicine before, we advise you to read this text carefully. The information may have been changed.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Xalatan is and what it is used for
2. Before you use Xalatan
3. How to use Xalatan
4. Possible side effects
5. How to store Xalatan
6. Further information
1. WHAT XALATAN IS AND WHAT IT IS USED FOR
Xalatan belongs to a group of medicines known as prostaglandin analogues. It works by increasing the natural outflow of fluid from inside the eye into the bloodstream.
Xalatan is used to treat conditions known as open angle glaucoma and ocular hypertension. Both of these conditions are linked with an increase in the pressure within your eye, eventually affecting your eye sight.
2. BEFORE YOU USE XALATAN
Xalatan can be used in adult men and women (including the elderly) but is not recommended for use if you are less than 18 years of age.
Do not use Xalatan if you are:
• Allergic (hypersensitive) to latanoprost or any of the other ingredients of Xalatan (see section 6 for the list of ingredients in your medicine)
• Pregnant or trying to become pregnant
• Breast feeding
Take special care with Xalatan
Talk to your doctor or pharmacist before you take Xalatan if you think any of the following apply to you:
• If you are about to have or have had eye surgery (including cataract surgery)
• If you suffer from eye problems (such as eye pain, irritation or inflammation, blurred vision)
• If you know that you suffer from dry eyes
• If you have severe asthma or your asthma is not well controlled
• If you wear contact lenses. You can still use Xalatan, but follow the instruction for contact lens wearers in Section 3
Taking other medicines
Xalatan may interact with other medicines. Please tell your doctor or pharmacist if you are taking or have taken any other medicines including those medicines (or eye drops) obtained without a prescription.
Pregnancy
Do not use Xalatan when you are pregnant. Tell your doctor immediately if you are pregnant, think you are pregnant, or are planning to become pregnant.
Breast-feeding
Do not use Xalatan when you are breast-feeding.
Driving and using machines
When you use Xalatan you might have blurred vision, for a short time. If this happens to you, do not drive or use any tools or machines until your vision becomes clear again.
Important information about some of the ingredients of Xalatan
Xalatan contains a preservative called benzalkonium chloride. This preservative may cause eye irritation or disruption to the surface of the eye. Benzalkonium chloride can be absorbed by contact lenses and is known to discolour soft contact lenses. Therefore, avoid contact with soft contact lenses.
If you wear contact lenses, you should remove them before using Xalatan. After using Xalatan you should wait 15 minutes before putting your contact lenses back in. See the instructions for contact lens wearers in Section 3.
APPROVED
By potrickn at 2:48 pm, Sep 15, 2009
3. HOW TO USE XALATAN
Always use Xalatan exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The usual dosage for adults (including the elderly) is one drop once a day in the affected eye(s). The best time to do this is in the evening.
Do not use Xalatan more than once a day, because the effectiveness of the treatment can be reduced if you administer it more often.
Use Xalatan as instructed by your doctor until your doctor tells you to stop.
Contact lens wearers
If you wear contact lenses, you should remove them before using Xalatan. After using Xalatan you should wait 15 minutes before putting your contact lenses back in.
Instructions for use
1. Wash your hands and sit or stand comfortably.
2. Twist off the outer cap (which can be thrown away).
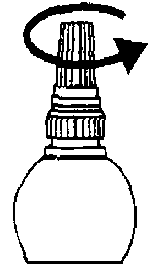
3. Unscrew the protective inner cap. The protective cap should be retained.
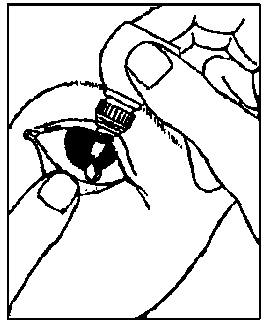
Use your finger to gently pull down the lower eyelid of your affected eye.
Place the tip of the bottle close to, but not touching your eye.
Squeeze the bottle gently so that only one drop goes into your eye, then release the lower eyelid.
Press a finger against the nose or inner corner of the affected eye. Hold for 1 minute whilst keeping the eye closed.
Repeat in your other eye if your doctor has told you to do this.
Put the protective inner cap back on the bottle.
If you use Xalatan with other eye drops
Wait at least 5 minutes between using Xalatan and taking other eye drops
If you use more Xalatan than you should
If you put too many drops into your eye, you may experience some minor irritation in your eye and your eyes may water and turn red, this should pass, but if you are worried contact your doctor for advice.
Contact your doctor as soon as possible if you swallow Xalatan accidentally.
If you forget to use Xalatan
Carry on with the usual dosage at the usual time. Do not take a double dose to make up for the dose you have forgotten. If you are unsure about anything talk to your doctor or pharmacist.
If you stop using Xalatan
You should speak to your doctor if you want to stop taking Xalatan.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Xalatan can cause side effects, although not everybody gets them.
Very common effects (likely to affect more than 1 in 10 people):
• A gradual change in your eye colour by increasing the amount of brown pigment in the coloured part of the eye known as the iris. If you have mixed-colour eyes (blue-brown, grey-brown, yellow-brown or green-brown) you are more likely to see this change than if you have eyes of one colour (blue, grey, green or brown eyes). Any changes in your eye colour may take years to develop although it is normally seen within 8 months of treatment. The colour change may be permanent and may be more noticeable if you use Xalatan in only one eye. There appears to be no problems associated with the change in eye colour. The eye colour change does not continue after Xalatan treatment is stopped.
• Redness of the eye.
• Eye irritation (a feeling of burning, grittiness, itching, stinging or the sensation of a foreign body in the eye).
• A gradual change to eyelashes of the treated eye and the fine hairs around the treated eye, seen mostly in people of Japanese origin. These changes involve an increase of the colour (darkening), length, thickness and number of your eye lashes.
Common effects (likely to affect less than 1 in 10 people):
• Irritation or disruption to the surface of the eye, eyelid inflammation (blepharitis) and eye pain.
Uncommon effects (likely to affect less than 1 in every 100 people):
• Eyelid swelling, dryness of the eye, inflammation or irritation of the surface of the eye (keratitis), blurred vision and conjunctivitis.
• Skin rash.
Rare effects (likely to affect less than 1 in every 1000 people):
• Inflammation of the iris, the coloured part of the eye (iritis/uveitis); swelling of the retina (macular oedema), symptoms of swelling or scratching/damage to the surface of the eye, swelling around the eye (periorbital oedema) misdirected eyelashes or an extra row of eyelashes.
• Skin reactions on the eyelids, darkening of the skin of the eyelids.
• Asthma, worsening of asthma and shortness of breath (dyspnoea).
Very rare effects (likely to affect less than 1 in 10,000 people):
• Worsening of angina in patients who also have heart disease. Chest pain.
Patients have also reported the following side-effects: headache, dizziness, palpitations, muscle pain and joint pain.
If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please contact you doctor or pharmacist.
5. HOW TO STORE XALATAN
Before Xalatan is first opened keep it in a fridge (between 2°C and 8°C / 36°F and 46°F), out of direct light.
Once the bottle has been opened, it is not necessary to put it in the fridge; Xalatan can be kept at normal room temperature (below 25°C / 77°F).
Each bottle should be thrown away four weeks from first opening.
The bottle label has an expiry date. Do not use after this date. The expiry date refers to the last day of that month.
Keep all medicines in a safe place, out of the reach and sight of children.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Xalatan contains
The active substance is 0.005% (50 microgrames/ml) latanoprost.
The other ingredients are: Benzalkonium chloride Ph Eur, Sodium chloride Ph Eur, Sodium dihydrogen phosphate monohydrate, Disodium phosphate anhydrous, Water for injections Ph Eur
What Xalatan looks and contents of the pack
Xalatan Eye Drops, Solution is a clear, colourless liquid.
Each bottle contains 2.5 ml of solution.
Manufacturer
This product is manufactured by: Pfizer Manufacturing Belgium NV, Puurs, Belgium.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd, Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: BR Lewis Pharmaceuticals Ltd, Kirk Sandall, Doncaster, DN3 1QR.
|POM| PL No: 08929/0033
Leaflet review and issue date (Ref): 04.09.09 Xalatan® is a registered trademark of Pfizer Ltd.
Page 2 of 2