Manx Healthcare Sore Throat Relief Spray 1.5Mg Oromucosal Spray
PATIENT INFORMATION LEAFLET - INFORMATION FOR THE USER
MANX Throat Relief Spray 1.5mg Oromucosal Spray
Benzocaine
Please read this leaflet carefully before you start using your medicine because it contains important information for you.
■ Keep this leaflet. You may need to read it again.
■ Ask your pharmacist if you need more information or advice.
■ You must contact a doctor if your symptoms worsen or do not improve after 3 days.
■ If any side effect occurs and it seems serious to you, or if you notice any side effect not listed in this leaflet, please tell your doctor or pharmacist.
What is in this leaflet
1. What Sore Throat Relief Spray is and what it is used for
2. Before you use Sore Throat Relief Spray
3. How to use Sore Throat Relief Spray
4. Possible side effects
5. How to store Sore Throat Relief Spray
6. Further information
1. What Sore Throat Relief Spray is and what it is used for
Manx Healthcare Sore Throat Relief Spray 1.5mg Oromucosal Spray (hereinafter referred to as Sore Throat Relief Spray) is used to relieve the pain of sore throats. The product contains benzocaine, which is a local anaesthetic. It has a fast action and works by numbing the pain of a sore throat.
2. Before you use Sore Throat Relief Spray
Do not use this medicine if:
■ you have had an allergic (hypersensitive) reaction such as rash, itching or wheezing to benzocaine or any of the other ingredients of this medicine (see section 6 for other ingredients)
■ you have any difficulty breathing, noisy breathing, or severe difficulty in swallowing
■ you have a rare blood condition called methaemoglobinaemia
■ you are pregnant or breast feeding.
Do not use in children 12 years and under. Consult your doctor or pharmacist if you have:
■ a headache, fever (raised temperature) or nausea for more than a few days
■ a sore throat for more than a few days, or if the throat is so sore that you cannot swallow
■ had an allergic reaction to any other local anaesthetic
■ had an allergic reaction to a sunscreen product.
Important information about some of the
ingredients in this product
This spray contains a small amount of ethanol
(alcohol), less than 100mg per dose.
3. How to use Sore Throat Relief Spray
This medicine is for use by adults and teenagers.
Please follow the handling instructions below:
■ Shake the container before use
■ Before use it may be necessary to press the plunger 2-3 times to activate spray. Do this away from your face, into a sink
■ Do not spray into eyes. If you accidentally spray into eyes, rinse them thoroughly with water.
Fig 1
Resting position (cannot be activated)
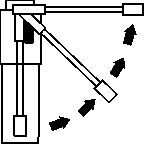
Fig 2
Raise nozzle from vertical to horizontal position
Fig 3
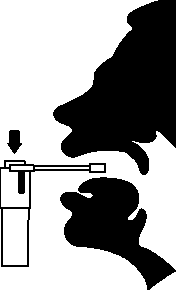
Hold your head upright and insert the nozzle well into your mouth. Hold your breath and press the plunger once to deliver a single metered spray.
(continued overleaf)
|
WIP URN |
010415-MST01-PIL-03 | |
|
APPROVED URN |
N/A | |
|
Job |
Sore Throat Relief Spray PIL - Side 1 | |
|
Size |
210 x 148mm | |
|
Date |
1 April 2015 | |
|
BOH Approval Date | ||
|
Saved as |
14123/03 PIL S1.ai M1 | |
|
Prints |
1 Colour - Black |
This leaflet was last revised in April 2015
Dosage:
Adults and Teenagers (13+):
■ Hold breath and spray twice to the back of the throat
■ Repeat every 2-3 hours if required
■ Do not use more than 8 times a day.
Do not use in children 12 years and under.
Do not use for more than 3 days in a row. If symptoms do not improve, consult your pharmacist or doctor.
You should experience temporary numbness in your throat after using the spray. This means this medicine is working. Avoid eating or drinking while your throat is numb.
If you use more of this medicine than you should If you spray more than you should, and you feel drowsy or unwell in any way, you must contact a doctor immediately.
4. Possible side effects
Like all medicines, Sore Throat Relief Spray can cause side effects, although not everybody gets them.
If you experience any of the following allergic reactions stop using the spray and seek immediate medical advice:
■ severe rash, itching, swelling of the face, lips or mouth
■ shortness of breath or difficulty breathing Methaemoglobinaemia (a very rare blood condition) has been reported with benzocaine use. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Sore Throat Relief Spray
Keep out of the sight and reach of children.
Do not store above 25°C.
Do not use the spray after the expiry date which is printed on the pack.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further Information
What Sore Throat Relief Spray contains Each metered dose (spray) of this medicine contains the active benzocaine 1.5mg. It also contains cetylpyridinium chloride, glycerin, ethanol, clove bud oil, peppermint, menthol, saccharin sodium 450, cremophor RH40 (polyethoxylated castor oil) and purified water.
What Sore Throat Relief Spray looks like and contents of the pack
The spray is a clear colourless to straw coloured solution available in a white aluminium can with a folding arm activator and cap.
Each can contains approximately sixty metered doses (sprays).
Marketing Authorisation Holder
Manx Pharma Ltd, Taylor Group House, Wedgnock Lane, Warwick, CV34 5YA, United Kingdom.
Manufacturer
Pharmasol Ltd, North Way, Walworth Industrial Estate, Andover, Hampshire, SP10 5AZ, UK.
Other Formats
To request a copy of this leaflet in Braille, large print or audio please call 01926 482511.
MANX
Healthcare
WIP URN: 010415-MST01 -PIL-03
|
WIP URN |
010415-MST01-PIL-03 | |
|
APPROVED URN |
N/A | |
|
Job |
Sore Throat Relief Spray PIL - Side 2 | |
|
Size |
210 x 148mm | |
|
Date |
1 April 2015 | |
|
BOH Approval Date | ||
|
Saved as |
14123/03 PIL S2.ai M1 | |
|
Prints |
1 Colour - Black |