Manx Healthcare Sore Throat Relief Spray 1.5Mg Oromucosal Spray
1. NAME OF THE MEDICINAL PRODUCT
Manx Healthcare Sore Throat Relief Spray 1.5 mg Oromucosal Spray
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Benzocaine 1.5 mg per actuation. For excipients please see 6.1.
3. PHARMACEUTICAL FORM
Oromucosal spray
Direct application to the throat by spraying Clear, colourless to straw coloured liquid
4. CLINICAL PARTICULARS
4.1 Therapeutic Indications
Symptomatic relief of sore throat pain
4.2 Posology and Method of Administration
Adults and children 13 years and over: Administer 2 sprays (3 mg) to the back of the throat. Repeat every two to three hours up to a maximum of 8 doses per day.
This product is contraindicated in children 12 years and under.
Method of administration: oromucosal
Hold breath and spray to the back of the throat.
Do not use in a patient who is unable to hold their breath whilst spraying.
Before first use or after prolonged storage press the plunger 2 - 3 times to activate the spray. Do this away from the face, into a sink.
Instructions for use of Manx Healthcare Sore Throat Relief Spray 1.5 mg Oromucosal Spray

Fig. 1
Resting position (cannot be activated)
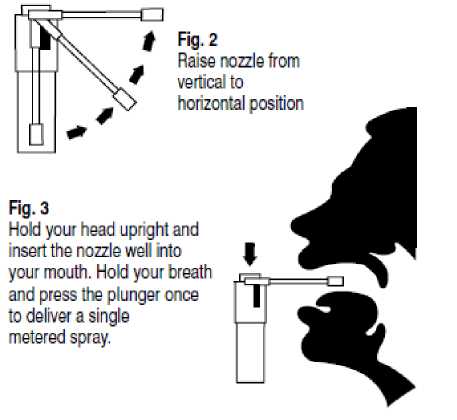
Pressing the plunger once delivers a single metered spray.
4.3 Contra-Indications
Children 12 years and under
Epiglottitis
Known hypersensitivity to benzocaine or any of the other ingredients
Methaemoglobinaemia
4.4 Special Warnings and Precautions for Use
Do not administer to children 12 years and under. Do not use for more than 3 consecutive days.
Do not spray into eyes.
If sore throat is severe or persistent, or accompanied by fever, headache or nausea consult your doctor.
Caution should be exercised in the use of this product if there have been previous allergic reactions with other local anaesthetics or sunscreen products.
You should experience temporary numbness in your throat after using the spray. This indicates that the product is working. Avoid eating or drinking as long as the numbness lasts.
Labelling will include the following information:
Do not use if you have difficulty in breathing, noisy breathing or severe difficulty in swallowing.
Do not use if you have been told that you have a rare blood condition called methaemoglobinaemia.
4.5 Interactions with other medicinal products and other forms of Interaction
None known
4.6 Pregnancy and Lactation
Animal studies are insufficient with respect to effects on pregnancy and lactation. The potential risk for humans is unknown. Therefore Manx Healthcare Sore Throat Relief Spray 1.5 mg Oromucosal Spray is not recommended during pregnancy or breastfeeding.
4.7 Effects on ability to drive and use machines
None.
4.8 Undesirable effects
Allergic reactions have been reported very occasionally with benzocaine. There have been occasional reports of temporary breathing difficulty, face or mouth swelling. Methaemoglobinaemia has been reported with benzocaine use.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is
important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
4.9 Overdose
Pronounced reversible anaesthesia would be observed. No systemic adverse effects are expected due to the poor systemic absorption and low administered dose of benzocaine.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic Properties
ATC code: R02AD01
Benzocaine is a surface anaesthetic of the ester type. The mode of action is a reversible inhibition of the flux of sodium and potassium ions through the axonal membranes of peripheral pain receptors. As a consequence, the depolarisation and propagation of nerve impulses are inhibited.
The onset of action on the mucous membranes is rapid due to the spray delivery of the anaesthetic direct to the site of action, rapid absorption, and the surface analgesic effect. The local anaesthesia induced by benzocaine is temporary but Manx Healthcare Sore Throat Relief Spray 1.5 mg Oromucosal Spray has not been tested for duration of action.
5.2 Pharmacokinetic properties
Benzocaine is absorbed into the mucosal membranes. After systemic absorption, which is neglible, the drug is thought to be metabolised to ethanol and aminobenzoic acid by plasma esterases. Aminobenzoic acid is excreted unchanged or conjugated with glycerine to amoniohippuric acid in the liver, the metabolites and unchanged benzocaine are excreted in the urine.
5.3 Preclinical Safety Data
No animal data are available on Manx Healthcare Sore Throat Relief Spray
1.5 mg Oromucosal Spray Non-clinical studies on benzocaine showed local irritation
and sensitisation, and methaemoglobinaemia at high doses in some species.
PHARMACEUTICAL PARTICULARS
6.
6.1 List of Excipients
Cetylpyridinium chloride Glycerin,
Ethanol Clove bud oil Levomenthol Sodium saccharin Peppermint Cremophor RH40 Purified water
6.2 Incompatibilities
None.
6.3 Shelf life
24 months.
6.4 Special Precautions for Storage
Do not store above 25 °C.
6.5 Nature and contents of container
White aluminium spray container with metering pump and high density polyethylene cap, and polypropylene and nitrilic rubber nozzle.
Pack size: not less than 7.3 g (approximately 60 metered doses).
To help protect the environment, do not dispose of this medicine via wastewater or household waste. Ask a pharmacist for advice on disposal.
7
Manx Pharma Limited Taylor Group House Wedgnock Lane Warwick CV34 5YA United Kingdom
8
9
09/06/2015