Mysodelle 200 Micrograms Vaginal Delivery System
Package leaflet: Information for the user
Q
FERRING
Mysodelle® 200 micrograms vaginal delivery system
Misoprostol
to
I—
O
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this
See section 4.
to
X
LU
I—
o
What is in this leaflet
1. What Mysodelle is and what it is used for
2. What you need to know before you are given Mysodelle
3. How you are given Mysodelle
4. Possible side effects
5. How to store Mysodelle
6. Contents of the pack and other information
1. What Mysodelle is and what it is used for
Mysodelle contains the active substance misoprostol.
Mysodelle is used to help start the birth process from 36 weeks of pregnancy.
Misoprostol belongs to a group of medicines called prostaglandins. Prostaglandins have two actions during labour. One is to soften the mouth of the womb (cervix) so that the baby can more easily be born through the vagina. The second is to cause contractions to start, which will help push the baby out of the womb (uterus). There could be several reasons why you might need help to start this process. Ask your doctor if you want more information.
2. What you need to know before you are given Mysodelle Do not use Mysodelle:
- if you are allergic to misoprostol or any of the other ingredients of this medicine (listed in section 6)
- if labour has started
- if your baby is not in good health and/ or is distressed
- if oxytocic drugs (medicines used to facilitate birth) and/or other medicines to induce labour are being given (see “Warnings and precautions” and “Other medicines and Mysodelle” below)
- if you have had previous cervical or womb surgery including a previous Caesarean birth for any earlier babies
- if you have any womb abnormality such as “heart-shaped” uterus (bicornate uterus)
- if your placenta is covering the birth canal (placenta praevia) or if you have had any unexplained vaginal bleeding after the 24th week of this pregnancy
- if your baby is not in the correct position in the womb to be born naturally (fetal malpresentation)
- if you have any signs or symptoms of . of the
waters that surround your baby (chorioamnionitis), unless treatment has already been given
- if you are less than 36 weeks pregnant.
Warnings and precautions
Mysodelle should only be used under the supervision of an appropriate specialist.
Your doctor or nurse will carefully monitor womb activity, status of your baby and changes in the neck of the womb (cervix) when Mysodelle is in place.
Mysodelle can cause strong womb stimulation if left in place after onset of labour (see “If you use more Mysodelle than you should” below).
In case the womb contractions are prolonged or strong or your doctor or nurse is concerned for you or your baby, Mysodelle will be removed. If the womb contractions continue after removal of Mysodelle, tocolytic treatment may be given which will slow down your contractions.
The effects of Mysodelle have not been studied in women with severe pre-eclampsia (a condition where pregnant women suffer from high blood pressure, protein in the urine and possibly other complications).
Mysodelle has not been studied in women whose waters have been broken for more than 48 hours prior to insertion of Mysodelle. Please tell your doctor or if you think your waters might have broken (premature rupture of your membranes).
If you have an infection (Group B Streptococcus) that requires preventive antibiotic therapy, the antibiotic treatment may be given to you at the same time as Mysodelle or earlier so that you and your baby are treated before birth. If you know you have an infection, please tell your doctor or nurse.
If your doctor that treatment with oxytocin (medicine used to facilitate birth) should be started, Mysodelle must be removed by the doctor or nurse at least 30 minutes prior to oxytocin adminstration (see “Do not use Mysodelle” above and “Other medicines and Mysodelle” below).
A second dose of Mysodelle is not recommended, as the effects of a second dose have not been studied.
An increased risk of disseminated intravascular coagulation (severe bleeding) after delivery has been described in patients whose labour has been induced by any method.
There is no experience with the use of Mysodelle to start the birth process in women who are pregnant with more than one baby and there is no experience with the use of Mysodelle in women who have had more than 3 previous babies delivered vaginally after 24 weeks of pregnancy.
Mysodelle is only used if you have a medical reason for needing help to start the birth process.
Other medicines and Mysodelle
Tell your doctor or nurse if you are using, have recently used or might use any other medicines. Some other medicines may influence the effect of Mysodelle.
Mysodelle must not be given at the same time as oxytocic drugs (medicines used to facilitate birth) and/or other medicines to help start labour (see “Do not use Mysodelle” and “Warnings and precautions” above).
Pregnancy and breast-feeding
Mysodelle is used to help start labour from week 36 of the pregnancy. Mysodelle should not be used at other phases of pregnancy.
Misoprostol acid may be excreted in colostrum (the excreted by the breasts for the . 3 - 4 days after delivery) and breast milk, but the level and duration is expected to be very limited and should not hinder breast-feeding.
Fertility
Fertility will not be affected by use of Mysodelle to help start the birth process from 36 weeks of pregnancy.
Mysodelle contains Butylated hydroxyanisole
Mysodelle contains butylated hydroxyanisole which is used as an antioxidant that preserves the product. It is only present in trace amounts. Butylated hydroxyanisole can cause skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes.
3. How you are given Mysodelle
The recommended dose is one Mysodelle vaginal delivery system which contains 200 micrograms of misoprostol. The active ingredient, misoprostol, is released at an average rate of approximately 7 micrograms per hour over a 24 hour period.
Your doctor or nurse will place one Mysodelle in your vagina next to the neck of your womb (cervix). You will not do this yourself. Your doctor or nurse may coat Mysodelle with a small amount of lubricating jelly before putting it in place. Mysodelle can easily be pulled out by the doctor or nurse when it is time to remove it.
You will be lying down during this procedure and you will have to stay that way for about 30 minutes after insertion of Mysodelle.
Once placed in the vagina, Mysodelle takes up moisture and slowly releases misoprostol.
When using the toilet, please use caution to avoid removing Mysodelle by mistake. Tell the doctor or nurse if Mysodelle falls out at any time.
The doctor or nurse will decide how long Mysodelle will be kept in place, depending on your progress. Mysodelle can be left in place for up to 24 hours.
Your doctor or nurse will remove Mysodelle
- when labour starts
- if your contractions are too strong or prolonged
- if your baby becomes distressed
- if 24 hours have elapsed since insertion
If Mysodelle falls out, it will not be replaced.
On removal of the product from the vagina, Mysodelle will have swollen to 2-3 times of its original size and be flexible.
Use in children and adolescents
Mysodelle has not been studied in pregnant women less than 18 years of age.
O
_i
LU
CO
X
I-
I—
X
LU
I-
o
CO
X
I-
I—
X
LU
I-
If you use more Mysodelle than you should
If Mysodelle is left in place after onset of active labour it may lead to increasing contractions or the baby may become distressed. Mysodelle will then be removed immediately by your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common: may affect up to 1 in 10 people:
• The unborn baby’s heart rate changes during labour which may be a reason for concern (foetal heart rate disorder)
• The mother’s womb contracts too frequently and the unborn baby’s heart rate may be affected which may be a reason for concern (abnormal labour affecting foetus)
• The baby has a bowel movement in the womb which may be a reason for concern (meconium in amniotic fluid)
• The mother’s womb contracts too frequently which may be a reason for concern (uterine contractions abnormal).
Uncommon: may affect up to 1 in 100 people:
• Brain effects in the baby due to not enough oxygen (hypoxic-ischaemic encephalopathy)
• The baby has ' breathing immediately after birth
(neonatal respiratory depression; neonatal respiratory distress syndrome; transient tachypnoea of the newborn)
• Nausea
• Vomiting
• Rash
• Unexpected bleeding from the vagina before delivery (antepartum haemorrhage)
• Increased acidity in the baby’s blood (foetal acidosis)
• Excessive vaginal bleeding after birth (postpartum haemorrhage)
• The placenta separates from the wall of the womb before the birth of the baby (premature separation of placenta)
• A contraction that lasts too long and may be a reason for concern (uterine hypertonus)
• Itching of the genital area (pruritus genital)
• Overall newborn condition depressed at birth (Apgar score low)
• Increase in blood pressure
• Tearing of the womb (uterine rupture).
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any side effects not listed in this You can also
report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland
Pharmacovigilance Section
Irish Medicines Board
Kevin O’Malley House
Earlsfort Centre
Earlsfort Terrace
IRL - Dublin 2
Tel: +353 1 6764971
Fax: +353 1 6762517
Website: www.imb.ie
e-mail: imbpharmacovigilance@imb.ie
5. How to store Mysodelle
Keep this medicine out of the sight and reach of children.
Store in a freezer (-10 to -25°C). No thawing is required prior to use.
Do not use this medicine after the expiry date which is stated on the foil and the carton after EXP The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or househol d waste. Your doctor or nurse should throw away or dispose of medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Mysodelle contains
- The active substance is misoprostol.
Each vaginal delivery system contains 200 micrograms of misoprostol and releases misoprostol at a mean rate of approximately 7 micrograms/hour over 24 hours.
- The other excipients are:
Cross-linked hydrogel polymer (comprised of macrogol,
1,2,6- hexanetriol and dicyclohexyl-methane-4,4’-diisocyanate)
butylated hydroxyanisole
polyester retrieval system (knitted polyester yarn)
What Mysodelle looks like and contents of the pack
Mysodelle contains a reservoir of 200 micrograms misoprostol. Mysodelle is a small rectangular shaped piece of plastic contained in a cloth mesh retrieval system. The plastic is a hydrogel polymer which swells in the presence of moisture to release a controlled amount of misoprostol. The retrieval system has a long tape which allows the doctor or nurse to remove it when they need to.
1 x 200 micrograms vaginal delivery system 5 x 200 micrograms vaginal delivery system 5 x 200 micrograms vaginal delivery system (multipack).
Each vaginal delivery system is contained within an individual foil sachet produced from an aluminium foil laminated strip containing a desiccant and packed in a carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Ferring Pharmaceuticals Ltd.
Drayton Hall, Church Road, West Drayton, UB7 7PS, (UK) PL 03194/0112
Ferring Ireland Ltd.
United Drug House, Magna Drive, Magna Business Park, Citywest Road, Dublin 24, Ireland PA 1009/025/001
Manufacturer
Ferring Controlled Therapeutics Limited 1 Redwood Place, Peel Park Campus, East Kilbride Scotland, G74 5PB
This medicinal product is authorised in the Member States of the EEA under the following names:
Misodel: Austria, Bulgaria, Cyprus, Czech Republic, Denmark,
Finland, France, Germany, Greece, Hungary, Iceland, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Spain and Sweden. Mysodelle: Belgium, Estonia, Ireland, Italy, Luxemburg, Slovenia, and UK.
This leaflet was last revised in 10/2013.
Mysodelle, FERRING and the FERRING Logo are trade marks of Ferring B.V. © 2013 Ferring B.V.
The following information is intended for healthcare professionals only.
Mysodelle is supplied in an individual aluminium foil sachet. There is a “tear mark” on one side of the foil sachet. Open the package along the tear mark across the top of the sachet. Do not use scissors or other sharp objects which may cut the retrieval system.
Place Mysodelle high in the posterior vaginal fornix (Figure a). To ensure that Mysodelle remains in situ, it should be turned 90o so that it lies transversely in the posterior fornix of the vagina (Figure b). Water-soluble lubricants may be used to aid insertion when necessary.
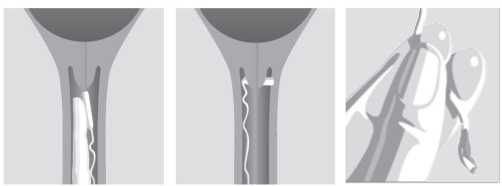
Figure a. Figure b. Figure c.
After Mysodelle has been inserted, the withdrawal tape may be cut with scissors always ensuring there is sufficient tape outside the vagina to allow removal.
The patient is to remain in bed for 30 minutes after insertion, but may be ambulatory thereafter. Take care not to inadvertently remove Mysodelle during toileting and vaginal examinations. Mysodelle is removed by gently pulling the tail of the retrieval system (Figure c).
The vaginal delivery system should NEVER be removed from the retrieval system.
Mysodelle is a controlled release formulation that swells in the presence of moisture, causing drug release to occur. During insertion, Mysodelle will swell to 2-3 times its original size and be pliable. After removal, ensure that the entire product (insert and retrieval system) has been removed from the vagina.