Mysodelle 200 Micrograms Vaginal Delivery System
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Mysodelle 200 micrograms vaginal delivery system.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Mysodelle contains 200 micrograms misoprostol.
Misoprostol is released in vivo at a mean rate of approximately 7 micrograms/hour over a period of 24 hours. Drug release continues as long as Mysodelle is in the vagina.
Excipients with known effect: 0.13 mg butylated hydroxyanisole per dose (see Section 4.4).
For the full list of excipients, see Section 6.1.
3 PHARMACEUTICAL FORM
Vaginal delivery system.
The polymer insert is contained within a retrieval system consisting of an inert woven polyester pouch and tail. The polymer insert is rectangular in shape with radiused corners, is buff coloured, semi-transparent, non-biodegradable and measures approximately 30 mm in length, 10 mm in width and 0.8 mm in thickness. Mysodelle swells in the presence of moisture.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Mysodelle is indicated for induction of labour in women with an unfavourable cervix, from 36 weeks gestation, in whom induction is clinically indicated.
4.2 Posology and method of administration
Posology
Mysodelle 200 micrograms is a controlled release formulation that releases
misoprostol at a rate of approximately 7 micrograms/hour over a period of 24
hours.
The maximum recommended dose is one Mysodelle vaginal delivery system
(200 micrograms).
Remove Mysodelle
- at the onset of active labour (progressive cervical dilatation to 4 cm with any frequency of contractions or rhythmic, firm, adequate quality uterine contractions causing progressive cervical change occurring at a frequency of 3 or more in 10 minutes and lasting 45 seconds or more)
- if uterine contractions are prolonged or excessive
- if there is evidence of fetal compromise or
- if 24 hours have elapsed since insertion.
If Mysodelle falls out, do not replace it.
In case of subsequent administration of oxytocin, a waiting period of at least 30 minutes is recommended following the removal of the vaginal delivery system (see Section 4.5).
Paediatric population
The safety and efficacy of Mysodelle in pregnant women aged less than 18 years has not been established.
No data are available.
Method of administration
Mysodelle should only be administered by trained obstetric personnel in a hospital setting where facilities for continuous fetal and uterine monitoring is available. The condition of the cervix should be assessed carefully before Mysodelle is used. After insertion, uterine activity and fetal condition must be carefully monitored.
Mysodelle is supplied in an individual aluminium foil sachet, and must be stored in the freezer. No thawing is required prior to use.
There is a “tear mark” on one side of the foil sachet. Open the package along the tear mark across the top of the sachet. Do not use scissors or other sharp objects which may cut the retrieval system.
Place Mysodelle high in the posterior vaginal fornix (Figure a). To ensure that Mysodelle remains in situ, it should be turned 90o so that it lies transversely in the posterior fornix of the vagina (Figure b). Water-soluble lubricants may be used to aid insertion when necessary.
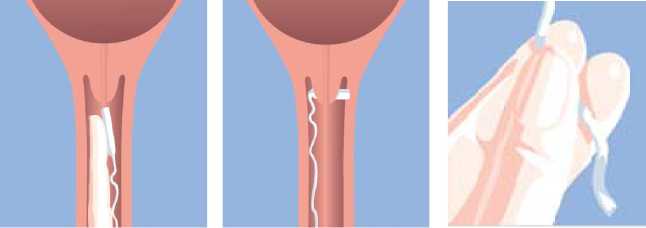
Figure a. Figure b. Figure c.
After the vaginal delivery system has been inserted, the withdrawal tape may be cut with scissors always ensuring there is sufficient tape outside the vagina to allow removal.
The patient is to remain in bed for 30 minutes after insertion, but may be ambulatory thereafter. Take care not to inadvertently remove Mysodelle during toileting and vaginal examinations.
Removal
Mysodelle is removed by gently pulling the tail of the retrieval system (Figure c).
The vaginal delivery system should NEVER be removed from the retrieval system.
Mysodelle is a controlled release formulation that swells in the presence of moisture, causing drug release to occur. During insertion, Mysodelle will swell to 2-3 times its original size and be pliable. After removal, ensure that the entire product (insert and retrieval system) has been removed from the vagina.
4.3 Contraindications
Mysodelle is contraindicated:
• When there is hypersensitivity to the active substance or to any of the excipients listed in Section 6.1
• When labour has started
• When there is suspicion or evidence of fetal compromise prior to induction (e.g., failed non-stress or stress test, meconium staining or diagnosis or history of non-reassuring fetal status)
• When oxytocic drugs and/ or other labour induction agents are being given (see section 4.4)
• When there is suspicion or evidence of uterine scar resulting from previous uterine or cervical surgery, e.g. caesarean delivery
• When there is uterine abnormality (e.g. bicornate uterus)
• When there is placenta praevia or unexplained vaginal bleeding after 24 weeks gestation with this pregnancy
• When there is fetal malpresentation
• When there are signs or symptoms of chorioamnionitis, unless adequate prior treatment has been instituted
• Before week 36 of gestation.
4.4 Special warnings and precautions for use
Mysodelle can cause excessive uterine stimulation if left in place after onset of active labour (see Section 4.9).
If uterine contractions are prolonged or excessive, or there is a clinical concern for the mother or baby, remove the vaginal delivery system. If excessive uterine contractions continue after drug removal, tocolytic treatments should be considered.
In women with pre-eclampsia, evidence or suspicion of fetal compromise should be ruled out (refer to Section 4.3). Pregnant women with severe preeclampsia marked by Haemolytic anaemia; Elevated Liver enzymes; Low Platelet count (HELLP) syndrome, other end organ affliction or CNS findings other than mild headache were not studied in the pivotal Phase III trial (Miso-Obs-303; The EXPEDITE Study).
Mysodelle has not been studied in women whose membranes have been ruptured for more than 48 hours prior to the insertion of Mysodelle.
For women with positive Group B Streptococcus status requiring prophylactic antibiotics, careful consideration should be given regarding timing of antibiotic therapy in order to achieve adequate protection. In the pivotal Phase III study (Miso-Obs-303; The EXPEDITE Study), the shortest observed time to any delivery was 2.95 hours.
Remove Mysodelle before oxytocin administration is initiated. Wait at least 30 minutes after removing Mysodelle before initiating oxytocin (see Sections 4.2, 4.3 and 4.5).
Mysodelle has only been studied in singleton pregnancies with cephalic presentation. No studies in multiple pregnancies have been performed. Mysodelle has not been studied in women with more than 3 previous vaginal deliveries after 24 weeks gestation.
Mysodelle should be used only when induction of labour is clinically indicated.
Mysodelle should be used with caution in patients with modified bishop score (mBS) >4.
A second dose of Mysodelle is not recommended, as the effects of a second dose have not been studied.
An increased risk of post-partum disseminated intravascular coagulation has been described in patients whose labour has been induced by any physiological or pharmacological method.
Butylated hydroxyanisole is used as an antioxidant in the cross-linked hydrogel polymer. It is only present in trace amounts in the final drug product. Butylated hydroxyanisole can cause skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with Mysodelle.
Concurrent use of oxytocic drugs or other labour induction agents is contraindicated due to the potential of increased uterotonic effects (see Sections 4.2, 4.3 and 4.4).
Other prostaglandin-containing products were given to subjects if needed in the clinical trials following removal of Mysodelle without apparent ill effect. A one-hour waiting period following removal of Mysodelle was utilised prior to allowing these products.
4.6 Fertility, pregnancy and lactation Fertility
From fertility and early embryonic development studies in rats, there is evidence of a possible adverse effect of misoprostol on implantation, however this is not relevant for the indicated clinical use of Mysodelle (see Section 5.3).
Pregnancy
Mysodelle has been studied in pregnant women > 36 weeks gestation. Mysodelle should not be used prior to 36 weeks of gestation (see Section 4.3).
Breast-feeding
No studies have been performed to investigate the amount of misoprostol acid in colostrum or breast milk following the use of Mysodelle.
Misoprostol acid has been detected in human milk following oral administration of misoprostol in tablet form.
After removal of Mysodelle, the median half-life in plasma of misoprostol acid is approximately 40 minutes. After five half-lives, i.e., approximately 3 hours, the misoprostol acid levels in the maternal plasma are negligible. Misoprostol acid may be excreted in colostrum and breast milk, but the level and duration is expected to be very limited and should not hinder breastfeeding. With Mysodelle, no effects on the breastfed newborns have been observed in the clinical development programme.
4.7 Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
Clinical Studies Experience Summary of the safety profile
The adverse reaction profile in Table 1 is based upon five clinical studies conducted with Mysodelle in 874 pregnant women at term gestation. The most common adverse reactions are uterine contractions abnormal, foetal heart rate disorder and abnormal labour affecting foetus.
|
System Organ Class |
Very common (>1/10) |
Common (>1/100 to <1/10) |
Uncommon (>1/1,000 to <1/100) |
|
Nervous system disorders |
Hypoxic-ischaemic encephalopathy | ||
|
Cardiac disorders |
Foetal heart rate disorder*' | ||
|
Respiratory, thoracic and mediastinal disorders |
Neonatal respiratory depression Neonatal respiratory distress syndrome Transient tachypnoea of the newborn | ||
|
Gastrointestina l disorders |
Nausea Vomiting | ||
|
Skin and subcutaneous tissue disorders |
Rash | ||
|
Pregnancy, puerperium and perinatal conditions |
Abnormal labour affecting foetus*"* Meconium in amniotic fluid Uterine contractions abnormal*** |
Antepartum haemorrhage * Foetal acidosis Postpartum haemorrhage Premature separation of placenta Uterine hypertonus | |
|
Reproductive system and breast disorders |
Pruritus genital | ||
|
Investigations |
Apgar score low* Blood pressure increased | ||
|
Injury, poisoning and procedural complications |
Uterine rupture |
Table includes adverse reactions from Studies Miso-Obs-002, Miso-Obs-003, Miso-Obs-204, Miso-Obs-205 and Miso-Obs-303 (The EXPEDITE Study)
* Neonatal adverse reactions.
^Foetal heart rate disorder was reported as fetal heart rate abnormalities, fetal bradycardia, fetal tachycardia, unexplained absence of normal variability, fetal heart rate decreased, fetal heart rate deceleration, early or late decelerations, variable decelerations, prolonged decelerations.
^Abnormal labour affecting foetus was reported as uterine tachysystole or uterine hypertonus with fetal heart rate disorder.
^Uterine contractions abnormal was reported as uterine tachysystole.
In the pivotal Mysodelle study (Miso-Obs-303: The EXPEDITE Study), neonates were followed for the first month after delivery for hospital admission or emergency room visits. No adverse reactions were reported following hospital discharge.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
There is no experience with the use of more than one application of Mysodelle. The controlled release formulation and ability to remove Mysodelle thereby stopping misoprostol delivery limits the risk of overdose. Accidentally leaving Mysodelle in place after onset of active labour may lead to symptoms of prostaglandin overdose (excessive uterine stimulation). If this occurs, remove Mysodelle and manage in accordance with local protocol.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other gynaecologicals, oxytocics, prostaglandins, ATC-code: G02AD06.
Mechanism of action
Misoprostol is a synthetic analogue of Prostaglandin Ei (PGEi), a naturally occurring oxytocic compound. Prostaglandins of the F and E series have been shown to increase collagenase activity in rabbit uterine cervix fibroblasts in vitro and to cause cervical ripening and uterine contraction in vivo. These pharmacodynamic effects are considered to be the mechanism of action relevant for the clinical effect of Mysodelle.
PGE analogues also have a number of other effects, e.g. relaxation of bronchial and tracheal muscles, increase of mucus secretion and decrease of acid and pepsin secretion in the stomach, increase of renal blood flow, increase of circulating concentrations of adrenocorticotropic hormone and prolactin. These_pharmacodynamic effects are considered to be of no clinical importance with the short treatment.
Clinical efficacy and safety
The phase III pivotal study, (Miso-Obs-303: The EXPEDITE study), was a double-blind, randomised, multicentre study conducted in the US with 1,358 pregnant women. The study compared the efficacy and safety of Mysodelle to the 10 mg dinoprostone vaginal delivery system (PROPESS®). Nulliparous and parous women with an unfavourable cervix (modified Bishop score < 4) were randomly assigned to receive Mysodelle or PROPESS® for up to 24 hours treatment. The co-primary efficacy endpoint of the study was time to vaginal delivery and the co-primary safety endpoint was the incidence of Caesarean deliveries.
Table 2 presents the key primary and secondary data endpoints from this study.
Table 2 Miso-Obs-303: The EXPEDITE Study Key Endpoint Results
|
Mysodelle 200 mcg (N=678) |
PROPESS® 10mg (N=680) |
p-value | |
|
Median time to Vaginal Delivery of Neonate (hours) |
** 21.5h |
** 32.8h |
p < 0.001 |
|
Nulliparous Subjects |
29.2 h (n=441) |
43.1 h (n=451) |
p < 0.001 |
|
Parous Subjects |
13.4 h (n=237) |
20.1 h (n=229) |
p < 0.001 |
|
Incidence of Caesarean Delivery (n %) |
176 (26.0%) |
184 (27.1%) |
p = 0.646 |
|
Nulliparous Subjects |
152 (34.5%) |
168 (37.3%) |
p = 0.386 |
|
Parous Subjects |
24 (10.1%) |
16 (7.0%) |
p = 0.226 |
|
Median time to Overall Delivery of the Neonate (Vaginal and Caesarean) (h) |
18.3hf |
27.3hf |
p < 0.001 |
|
Overall Median time to Onset of Active Labour (hours) |
12.1hn |
18.6hn |
p < 0.001 |
|
Overall number of Subjects who received Pre-delivery Oxytocin [n (%)] |
324 (48.1%) (N=674) |
497 (74.1%) (N=671) |
p < 0.001 |
* Subjects who had a caesarean delivery, were discharged prior to delivery or withdrew consent during the first hospitalisation were censored using the longest time interval from study drug administration to caesarean delivery or to labour and delivery discharge (Kaplan Meier estimates).
** Summary of median time to vaginal delivery (only subjects who delivered vaginally): Mysodelle, 200 mcg: 16.6 h; PROPESS® 10 mg: 25.1 h
f Summary of median time to any delivery: Mysodelle, 200 mcg: 18.2 h; PROPESS® 10 mg: 27.2 h
Summary of median time to onset of active labour: Mysodelle, 200 mcg: 12.0 h; PROPESS® 10 mg: 18.0 h
Paediatric population
The European Medicines Agency has waived the obligation to submit results of clinical studies with Mysodelle in all subsets of the paediatric population in labour induction, in the granted indication (See Section 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Misoprostol, an ester, is rapidly metabolised to its active metabolite misoprostol acid. Only misoprostol acid is detectable in plasma. The acid is further metabolised to inactive dinor and tetranor acid metabolites prior to excretion in the urine.
In non-pregnant women, the Mysodelle vaginal delivery system has a controlled mean in vivo release rate of approximately 7 micrograms/ hour over a period of 24 hours. In a study of 24 pregnant women at term gestation, a
median Cmax of 45.8 pg/mL with a median Tmax of 4 hours was observed. Median terminal half-life (after removal of the insert) was approximately 40 minutes.
The serum protein binding of misoprostol acid is less than 90% and concentration independent at therapeutic doses.
5.3 Preclinical safety data
The active component in Mysodelle, misoprostol, revealed no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
No teratogenic effects of misoprostol were observed in rats at dosages up to 10 mg/kg/day. In rabbits, an increase in fetuses with extra ribs was observed at a dosage of 1 mg/kg/day, probably associated with maternal toxicity at this dose level. At near lethal dose levels to the fetal mice, various fetal defects were observed. There is evidence of a possible adverse effect of misoprostol on implantation and the No Observed Adverse Effect Level was found to be 0.4 mg/kg/day in fertility and early embryonic development studies in rats. The above mouse and rat findings are of no concern for Mysodelle as it is contraindicated for use prior to 36 weeks of gestation.
Peri/post natal toxicity studies in rats identified a no-effect dosage for effects of oral misoprostol on reproductive parameters to 1.0 mg/kg/day. By comparing exposure in rat and human kinetic studies, a safety factor of 20 is established for Mysodelle delivered as a 200 mcg dose in the form of a miniature version of the misoprostol vaginal delivery system.
There was no evidence of local irritation in the vagina or cervix following administration of Mysodelle to pregnant rats.
There are no hazards for humans regarding systemic toxicity for the hydrogel polymers, the polyester retrieval system and excipients based on conventional in vitro and in vivo testing and published toxicity data. Finally, the hydrogel polymers and polyester retrieval system are comprised of inert compounds with good local tolerability.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Cross-linked hydrogel polymer (comprised of macrogol, 1,2,6- hexanetriol and dicyclohexyl-methane-4,4’-diisocyanate).
Butylated Hydroxyanisole
Polyester retrieval system (knitted polyester yarn)
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years.
6.4 Special precautions for storage
Store in a freezer (-10 to -25°C). No thawing is required prior to use.
6.5 Nature and contents of container
1 x 200 micrograms vaginal delivery system 5 x 200 micrograms vaginal delivery system 5 x 200 micrograms vaginal delivery system (multipack).
Each vaginal delivery system is contained within an individual foil sachet produced from an aluminium foil laminate strip containing a desiccant and packed in a carton.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Mysodelle should be removed from the freezer and taken out of the laminated aluminium foil sachet just prior to insertion.
Any unused medical product or waste material should be disposed of in accordance with local requirements. The whole product should be disposed following removal.
7 MARKETING AUTHORISATION HOLDER
Ferring Pharmaceuticals Ltd Drayton Hall Church Road West Drayton UB7 7PS, (UK)
8 MARKETING AUTHORISATION NUMBER(S)
PL 03194/0112
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
01/11/2013
10 DATE OF REVISION OF THE TEXT
01/11/2013