Nacl 0.45% W/V Kcl 0.15% W/V & Glucose 5% W/V Iv Infusion
PATIENT INFORMATION LEAFLET
Sodium Chloride 0.45% w/v, Potassium
Chloride 0.15% w/v, and Glucose 5% Intravenous Infusion
Sodium chloride, potassium chloride, glucose
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
The name of your medicine is Sodium Chloride 0.45% w/v. Potassium Chloride
0. 15. w/v, and Glucose 5% Intravenous Infusion, which will be referred to as NaCI/ KCI/glucose infusion throughout this leaflet.
In this leaflet;
1. What NaCI/KCl/glucose infusion is and what it is used for
2. Before you are given NaCI/KCl/giucose infusion
3. How you areqiven NaCI/KCl/qlucose infusion
4. Possible side effects
5. How to store NaCi/KCl/glucose infusion
6. Further information
1. WHAT NACL/KCL/GLUCOSE INFUSION IS AND WHAT IT IS USED FOR
NaCI/KCl/glucose infusion is a sterile solution that does not produce fever. NaCI/KCi/glucose infusion is used to maintain electrolyte (salt) balance after an operation. (You will need blood tests to make sure you do not receive too much potassium. This is a risk in patients with kidney failure.)
2. BEFORE YOU ARE GIVEN NACL/KCL/ GLUCOSE INFUSION
You may not be given NaCi/KCl/glucose infusion if you have any of the following conditions:
• Addison's disease (a disease which causes salt to be lost in the urine)
• Adrenal insufficiency (a problem affecting the adrenal giand)
• Kidney disease
• Little or no urine produced
• High potassium levels (can be detected by a test carried out by a doctor).
You may also not be able to receive dextrose (a sugar) infusion if you have a liver disorder.
Take special care with NaCI/KCl/glucose infusion;
Before you are given NaCl/KCl/glucose infusion, a solution containing no potassium may be given first to make sure your kidneys are working properly.
Taking other medicines Tell your doctor if you are
• receiving potassium-containing solutions.
• taking a potassium-sparing diuretic (water tablets such as amiloride)
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding Please tell your doctor if you are pregnant, think you are pregnant or breast-feeding.
Important information about some of the ingredients of NaCl/KCl/glucose infusion NaCI/KCl/giucose infusion contains sodium and potassium. This will be taken into consideration for patients on a controlled sodium or potassium diet or for patients with a kidney disorder.
NaCl/KCl/glucose infusion also contains glucose. If you have been told by your doctor that you have an intolerance to some sugars, tell your doctor.
3. HOW YOU ARE GIVEN NACL/KCL/ GLUCOSE INFUSION
NaCl/KCl/glucose infusion will be given to you as an infusion (into a vein) in hospital by a doctor or a nurse.
Adults;
The dose used will depend upon the patient's condition. The rate of infusion should be no more than 10-20 mmols of
Sodium Chloride 0.45% w/v, Potassium
Chloride 0.15% w/v, and Glucose 5% Intravenous Infusion
potassium per hour. The maximum daily dose of potassium is 200 mmols.
Children:
Children may need a reduced dose.
Elderly:
The elderly may need a reduced dose particularly if they have a heart or kidney disorder.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you are given more NaCI/KCI/glucose infusion than you should It is very unlikely, as your doctor will know the correct amount to use.
However, tell your doctor if you have any concerns,
4. POSSIBLE SIDE EFFECTS
Like all medicines, NaCI/KCI/glucose can cause side effects, although not everybody gets them.
The following side effects have been reported:
* lack of energy or interest,
* confusion,
* pins and needles,
* weakness,
* low blood pressure,
* disturbed heart rhythm, and sometimes heart attack.
A blood clot in the infused vein has also been reported.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor.
5. HOW TO STORE NACL/KCL/CLUCOSE INFUSION
Your doctor and hospital pharmacist are responsible for the correct storage, use and disposal of NaCI/KCI/glucose infusion. NaCI/KCI/glucose infusion should be stored below 25°C.
Do not use after the expiry date. Any unused solution should be discarded.
Keep out of the reach and sight of children.
6. FURTHER INFORMATION
What NaCi/KCl/glucose infusion contains: Sodium Chloride BP 0.45% w/v
Potassium Chloride BP 0.15% w/v
Glucose anhydrous BP 5.00% w/v
Water for Injections BP
What NaCI/KCI/glucose infusion looks like and contents of the pack.
NaCI/KCI/glucose infusion comes in PVC containers in the following sizes:
500 ml, 1000 ml
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer:
Terumo BCT Ltd.
Old Belfast Road Mil I brook Larne
Northern Ireland BT40 2SH United Kingdom
For any information about this medicinal product, please contact Marketing Authorisation Holder:
This leaflet was last approved in April 2012
I
s
i
NaCI (M5%w/v, KCI 0.15%w/v instructions for use
and Glucose 5%w/v Intravenous Infusion
Sterile, apyrogenic solutions for Intravenous Infusion
Visual Inspection
1 Ensure that She Infusion fluid compiles with the written prescription
2 If ANY fluid Is present Inside the overwrap, regard the Integrity of the pack as suspect and discard the solutio n.
3 Visually examine Ihc lluid which should be clear and Iree from particles. !f any pi (tides are present, regard the integrity ol the pack as suspect and discard the solution.
*1 Mead all precautionary notes and check the expiry dele.
5 Do NOT reconnect partially used bags.
Pack Preparation
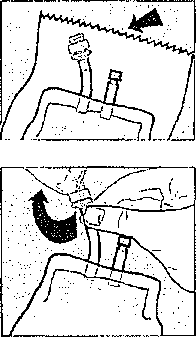
1 Remove the pack ham the protective overwrap by tearing downwards from the serrated edge.
2 Car el ul!>' straighten hunger and ports it necessary.
3 Tire sterile giving port is protected by a removable section ol the purl plug. To remove this protector, twist off the top section while holding the bottom section.
'1 Insert the giving set fully to produce a leak pronl connection and suspend the pack bom the inltrsiun stand
5 fume and regulate the giving set in accordance with the nninutacluret's Instructions. If giving set becomes blocked do not pump contents back into pack but replace equipment.
6 An air inlet is not required
7 Orscarrl any unused portion. Do not store partly used packs.
8 Discard all packs and equipment after use
Addition of Drugs
This pack has an additive port with a sell-sealing septum designed lot the addition ol drugs using a syringe and needle, or drug transfer device. This is the only site (or adding drugs.
Where possible, ready made solutions should be used. Check the drug/solution comp alibi lily. Drugs should only be added immediately prior to use and strict aseptic technique employed throughout the procedure. Do not add drugs until the hanger and ports have been straightened and the container inspected.
1 Swob the drug additive site with the appropriate anti-bacteria! lluid in accordance with current recommended practice and procedure.
2 Using a syringe and needle or drug transfer device, puncture the site and transfer the vial contents. Do not leave the needle or device in the site once the drug has been transletred. Removing the needle allows the rubber to reseol.
3 Ensure the adequate mixing of the contents before use. Observe the Iluto (or any changes which may indicate incompatibility,
4 Ensure that the addition has been recorded on the puck in accordance with routine procedure. The drug additive port should then be covered by an additive port cap to prevent lurther addition.
5 This presentation is latex Iree.
LEAF/332;3
Terumo BCT Ltd., Larne, BT40 2SH, U.K. Tel: (028) 2827 3631