Nacl 0.45% W/V Kcl 0.15% W/V & Glucose 5% W/V Iv Infusion
NaCI 0.45%w/v, KCI 0.15%w/v and Glucose 5%w/v Intravenous Infusion
INSTRUCTIONS FOR USE
The following information is intended for healthcare professionals only:
Sterile, apyrogenic solutions for Intravenous Infusion
Visual Inspection
1 Ensure that the infusion fluid complies with the written prescription.
2 If ANY fluid is present inside the overwrap, regard the integrity of the pack as suspect and discard the solution.
3 Visually examine the fluid which should be clear and free from particles. If any particles are present, regard the integrity of the pack as suspect and discard the solution.
4 Read all precautionary notes and check the expiry date.
5 Do NOT reconnect partially used bags.
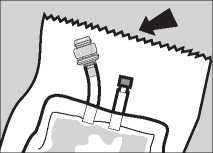
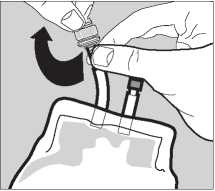
Pack Preparation
1 Remove the pack from the protective overwrap by tearing downwards from the serrated edge.
2 Carefully straighten hanger and ports if necessary.
3 The sterile giving port is protected by a removable section of the port plug. To remove this protector, twist off the top section while holding the bottom section.
4 Insert the giving set fully to produce a leak proof connection and suspend the pack from the infusion stand.
5 Prime and regulate the giving set in accordance with the manufacturer's instructions. If giving set becomes blocked do not pump contents back into pack but replace equipment.
6 An air inlet is not required.
7 Discard any unused portion. Do not store partly used packs.
8 Discard all packs and equipment after use.
Addition of Drugs
This pack has an additive port with a self-sealing septum designed for the addition of drugs using a syringe and needle, or drug transfer device. This is the only site for adding drugs.
Where possible, ready made solutions should be used. Check the drug/solution compatibility. Drugs should only be added immediately prior to use and strict aseptic technique employed throughout the procedure. Do not add drugs until the hanger and ports have been straightened and the container inspected.
1 Swab the drug additive site with the appropriate anti-bacterial fluid in accordance with current recommended practice and procedure.
2 Using a syringe and needle or drug transfer device, puncture the site and transfer the vial contents. Do not leave the needle or device in the site once the drug has been transferred. Removing the needle allows the rubber to reseal.
3 Ensure the adequate mixing of the contents before use. Observe the fluid for any changes which may indicate incompatibility.
4 Ensure that the addition has been recorded on the pack in accordance with routine procedure. The drug additive port should then be covered by an additive port cap to prevent further addition.
5 This presentation is latex free.
LEAF/3324
Terumo BCT Ltd., Larne, BT40 2SH, U.K. Tel: (028) 2827 3631
PATIENT INFORMATION LEAFLET PATIENT INFORMATION LEAFLET
Sodium Chloride 0.45% w/v, Potassium Sodium Chloride 0.45% w/v, Potassium
Chloride 0.15% w/v, and Glucose 5% Intravenous Infusion Chloride 0.15% w/v, and Glucose 5% Intravenous infusion
Sodium chloride, potassium chloride, glucose
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is Sodium Chloride 0.45% w/v, Potassium Chloride
0. 15. w/v, and Glucose 5% Intravenous Infusion, which will be referred to as NaCl/ KCl/glucose infusion throughout this leaflet.
In this leaflet:
1 .What NaCI/KCl/glucose infusion is and what it is used for
2. What you need to know before you are given NaCl/KCl/glucose
3. How you are given NaCI/KCl/glucose infusion
4. Possible side effects
5. How to store NaCI/KCl/glucose infusion
6. Contents of the pack and other information
1. WHAT NACL/KCL/GLUCOSE INFUSION IS AND WHAT IT IS USED FOR
NaCI/KCl/glucose infusion is a sterile solution that does not produce fever. NaCI/KCl/glucose infusion is used to maintain electrolyte (salt) balance after an operation. (You will need blood tests to make sure you do not receive too much potassium. This is a risk in patients with kidney failure.)
2. WHAT YOU NEED TO KNOW BEFORE YOU ARE GIVEN NACL/KCL/GLUCOSE
You may not be given NaCI/KCl/glucose infusion if you have any of the following conditions:
• Addison's disease (a disease which causes salt to be lost in the urine)
• Adrenal insufficiency (a problem affecting the adrenal gland)
• Kidney disease
• Little or no urine produced
• High potassium levels (can be detected by a test carried out by a doctor).
You may also not be able to receive dextrose (a sugar) infusion if you have a liver disorder.
Warnings and precautions
Before you are given NaCI/KCl/glucose infusion, a solution containing no potassium may be given first to make sure your kidneys are working properly.
Other medicine and NaCl/KCl/glucose
Tell your doctor if you are
• receiving potassium-containing solutions.
• taking a potassium-sparing diuretic (water tablets such as amiloride)
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
Please tell your doctor if you are pregnant, think you are pregnant or breast-feeding.
Important information about some of the ingredients of NaCI/KCl/glucose infusion
NaCI/KCl/glucose infusion contains sodium and potassium. This will be taken into consideration for patients on a controlled sodium or potassium diet or for patients with a kidney disorder.
NaCI/KCl/glucose infusion also contains glucose. If you have been told by your doctor that you have an intolerance to some sugars, tell your doctor.
3. HOW YOU ARE GIVEN NACL/KCL/ GLUCOSE INFUSION
NaCI/KCl/glucose infusion will be given to you as an infusion (into a vein) in hospital by a doctor or a nurse.
Adults:
The dose used will depend upon the patient's condition. The rate of infusion should be no more than 10-20 mmols of potassium per hour. The maximum daily dose of potassium is 200 mmols.
Children:
Children may need a reduced dose.
Elderly:
The elderly may need a reduced dose particularly if they have a heart or kidney disorder.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you are given more NaCI/KCl/glucose infusion than you should
It is very unlikely, as your doctor will know the correct amount to use.
You may experience symptoms relating to low blood pressure and a disturbed heart rhythm. However, tell your doctor if you have any concerns. If you have any further questions on the use of this medicine, ask your doctor.
4. POSSIBLE SIDE EFFECTS
Like all medicines, NaCI/KCl/glucose can cause side effects, although not everybody gets them.
The following side effects have been reported:
• lack of energy or interest,
• confusion,
• pins and needles,
• weakness,
• low blood pressure,
• disturbed heart rhythm, and sometimes heart attack.
A blood clot in the infused vein has also been reported.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at:
www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE NACL/KCL/GLUCOSE INFUSION
Keep this medicine out of the sight and reach of children.
Your doctor and hospital pharmacist are responsible for the correct storage, use and disposal of NaCI/KCl/glucose infusion. NaCI/KCl/glucose infusion should be stored below 25°C.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of that month. Any unused solution should be discarded.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What NaCI/KCl/glucose infusion contains:
Sodium Chloride BP 0.45% w/v
Potassium Chloride BP 0.15% w/v
Glucose anhydrous BP 5.00% w/v
Water for Injections BP
What NaCI/KCl/glucose infusion looks like and contents of the pack.
NaCI/KCl/glucose infusion comes in PVC containers in the following sizes:
500 ml, 1000 ml
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer:
Terumo BCT Ltd.
Old Belfast Road
Millbrook
Larne
Northern Ireland BT40 2SH United Kingdom
For any information about this medicinal product, please contact Marketing Authorisation Holder:
This leaflet was last approved in June 2016