Nebido 1000Mg/4Ml Solution For Injection
0
Package leaflet: Information for the user
Nebido
1000 mg/4 ml, solution for injection
Testosterone undecanoate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
► Keep this leaflet. You may need to read it again.
► If you have any further questions, ask your doctor.
► This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
► If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
|
What is in this leaflet | |
|
1. |
What Nebido is and what it is used for |
|
2. |
What you need to know before you are given Nebido |
|
3. |
How to use Nebido |
|
4. |
Possible side effects |
|
5. |
How to store Nebido |
|
6. |
Contents of the pack and other information |
1. What Nebido is and what it is used for
Nebido contains testosterone, a male hormone, as the active ingredient. Nebido is injected into a muscle in your body. There it can be stored and gradually released over a period of time. Nebido is used in adult men for testosterone replacement to treat various health problems caused by a lack of testosterone (male hypogonadism). These should be confirmed by two separate blood testosterone measurements and also include clinical symptoms such as:
► impotence
► infertility
► low sex drive
► tiredness
► depressive moods
► bone loss caused by low hormone levels
2. What you need to know before you are given Nebido
Do NOT use Nebido
► if you are allergic to testosterone undecanoate or any of the other ingredients of this medicine (listed in section 6)
► if you have androgen-dependent cancer or suspected cancer of the prostate or of the breast
► if you have or had a liver tumour Nebido is not intended for use in women.
Nebido is not for use in children and adolescents. There is no data available on the use of Nebido in males under 18 years of age.

Warnings and precautions
Talk to your doctor before using Nebido if you have or have ever had:
► epilepsy
► heart, kidney or liver problems
► migraine
► temporary interruptions in your breathing during sleep (apnoea), as these may get worse
► cancer, as the level of calcium in your blood may need to be tested regularly
► blood clotting problems
► high blood pressure or if you are treated for high blood pressure, as testosterone may cause a rise in blood pressure.
If you are suffering from severe heart, liver or kidney disease, treatment with Nebido may cause severe complications in the form of water retention in your body sometimes accompanied by (congestive) heart failure.
The following blood checks should be carried out by your doctor before and during the treatment: testosterone blood level, full blood count.
If your liver is not working No formal studies have been performed in patients with liver impairment. You will not be prescribed Nebido if you have ever had a liver tumour (see “Do not use Nebido”). Elderly patients (65 years or older)
There is no need for your doctor to adjust the dose if you are over 65 (see “Medical examination/follow up").
Muscle building and drug tests
Nebido is not suitable for building muscles in healthy
individuals or for increasing physical strength.
Nebido might lead to positive results in drug tests.
Medical examination/Follow-up Male hormones may increase the growth of prostate cancer and enlarged prostate glands (benign prostatic hypertrophy). Before your doctor injects Nebido, he/she will examine you to check that you do not have prostate cancer. Your doctor will regularly examine your prostate and breast, especially if you are elderly. He/she will also take regular blood samples.
Following the use of hormonal substances such as androgen compounds, cases of benign (non-cancerous) and malignant (cancerous) liver tumours have been observed to occur. Other medicines and Nebido Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines, including medicines obtained without a prescription. The doctor may need to adjust the dose if you are using any of the following:
► the hormone ACTH or corticosteroids (used to treat various conditions such as rheumatism, arthritis, allergic conditions and asthma): Nebido may increase
the risk of water retention, especially if your heart and liver are not working properly
► blood-thinning tablets (coumarin derived oral anticoagulants) since this can increase the risk of bleeding. Your doctor will check the dose.
► medicines used to treat diabetes. It may be necessary to ^ adjust the dose of your blood sugar reducing medicine.
Like other androgens, testosterone may increase the effect of insulin.
Please be sure to inform your doctor if you suffer from a disturbance of blood clotting, because this is important for your doctor to know before deciding to inject Nebido.
Nebido may also affect the results of some laboratory tests (e.g. thyroid gland). Tell your doctor or the laboratory staff that you are using Nebido.
Pregnancy and breast-feeding
Nebido is not for use in women and must not be used in
pregnant or breast-feeding women.
Fertility
Treatment with high doses of testosterone preparations commonly may reversibly stop or reduce sperm production (see also under “Possible side effects").
Driving and using machines
Nebido has no observed effect on your ability to drive or use machines.
3. How to use Nebido
Your doctor will inject Nebido (1 ampoule / vial) very slowly into a muscle. He/she will give you the injections every 10 to 14 weeks. This is enough to maintain sufficient testosterone levels without leading to a build-up of testosterone in the blood.
Nebido is strictly for intramuscular injection. Special care will be taken to avoid injection into a blood vessel (see “Notes on handling the OPC (One-Point-Cut) ampoule”).
Start of treatment
Your doctor will measure your blood testosterone levels before starting treatment and during the early stages of treatment. Your doctor may give you the second injection after only six weeks in order to quickly reach the necessary testosterone level. This will depend on your symptoms and testosterone levels.
Maintaining your Nebido levels during treatment
The injection interval should always be within the recommended range of 10 to 14 weeks.
Your doctor will measure your testosterone levels regularly at the end of an injection interval to make sure it is at the right level. If the level is too low, your doctor may decide to give you injections more often. If your testosterone levels are high, your doctor may decide to give you injections less often. Do not miss your injection appointments. Otherwise, your optimum level of testosterone will not be maintained. If you think that the effect of Nebido is too strong or too weak, talk to your doctor.
If you use more Nebido than you should Symptoms of having too much Nebido include:
► irritability
► nervousness
0
—K
lead to signs and symptoms such as cough, dyspnoea, malaise, hyperhidrosis, chest pain, dizziness, paraesthesia, or syncope. These reactions may occur during or immediately after the injection and are reversible. Treatment is usually supportive, e.g. by administration of supplemental oxygen. Suspected anaphylactic reactions after Nebido injection have been reported.
Warnings
Careful and regular monitoring of the prostate gland and breast must be performed in accordance with recommended methods (digital rectal examination and estimation of serum PSA) in patients receiving testosterone therapy at least once yearly and twice yearly in elderly patients and at risk patients (those with clinical or familial factors).
The following information is intended for healthcare professionals only:
The solution for intramuscular injection is to be visually inspected prior to use and only clear solutions free from particles should be used.
The contents of an ampoule / vial are to be injected intramuscularly immediately after opening the ampoule / vial.
The medicinal product is for single use only and any unused solution should be discarded.
Administration
Special care must be given to avoid intravasal injection.
As with all oily solutions, Nebido must be injected strictly intramuscularly and very slowly. Pulmonary microembolism of oily solutions can in rare cases
Besides laboratory tests of the testosterone concentrations in patients on long-term androgen therapy the following laboratory parameters should be checked periodically: haemoglobin, haematocrit, and liver function tests and lipid profile.
In patients suffering from severe cardiac, hepatic or renal insufficiency or ischaemic heart disease, treatment with testosterone may cause severe complications characterised by oedema with or without congestive cardiac failure. In such case, treatment must be stopped immediately.
85065211_06.indd 1
05.04.2016 07:35:57
11:i
► weight gain
► long-lasting or frequent erections
Tell your doctor, if you have any of these. Your doctor will inject it less often or will stop treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects,
although not everybody gets them.
The most common side effects are acne and pain where
the injection is given.
Common side effects (may affect up to 1 in 10 patients):
► abnormally high levels of red blood cells
► weight gain
► hot flushes
► acne
► enlarged prostate and associated problems
► various reactions where the injection was given (e.g. pain, bruising, or irritation)
Uncommon side effects (may affect up to 1 in
100 patients):
► allergic reaction
► increased appetite, changes in blood test results (e.g. increased blood sugars or fats)
► depression, emotional disorder, insomnia, restlessness, aggression, or irritability
► headache, migraine, or tremor
► cardiovascular disorder, high blood pressure, or dizziness
► bronchitis, sinusitis, cough, shortness of breath, snoring, or voice problems
► diarrhoea, or nausea
► changes in liver test results
► hair loss, or various skin reactions (e.g. itching, reddening, or dry skin)
► joint pain, pain in limbs, muscle problems (e.g. spasm, pain or stiffness), or an increased creatine phosphokinase in the blood
► urinary tract disorders (e.g. decreased flow of urine, urinary retention, urge to pass urine at night)
► prostatic disorders (e.g. prostatic intraepithelial neoplasia, or hardening or inflammation of the prostate), changes in sexual appetite, painful testicles, painful, hardened or enlarged breasts, or increased levels of male and female hormones
► tiredness, general feeling of weakness, excessive sweating, or night sweats
Rare side effects (may affect up to 1 in 1000 patients):
► The oily liquid Nebido may reach the lungs (pulmonary microembolism of oily solutions) which can in rare cases lead to signs and symptoms such as cough, shortness of breath, feeling generally unwell, excessive sweating, chest pain, dizziness, “pins and needles”, or fainting. These reactions may occur during or immediately after the injection and are reversible.
Suspected anaphylactic reactions after Nebido injection have been reported.
In addition to the side effects listed above the following have been reported following treatment with preparations containing testosterone: nervousness, hostility, brief interruptions in breathing during sleep, various skin reactions including dandruff and oily skin, increased hair growth, more frequent erections, and very rare cases of yellowing of the skin and eyes (jaundice).
Treatment with high doses of testosterone preparations commonly stops or reduces sperm production, although this returns to normal after treatment ceases. Testosterone replacement therapy of poorly functioning testicles (hypogonadism) can in rare cases cause persistent, painful erections (priapism). High-dosed or long-term administration of testosterone occasionally increases the occurrences of water retention and oedema (swelling due to fluid retention).
For testosterone products in general a common risk of increased red blood cell count, haematocrit (percentage of red blood cells in blood) and haemoglobin (the component of red blood cells that carries oxygen), were observed by periodic blood tests.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Nebido
Keep this medicine out of the sight and reach of children. This medicinal product does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the carton and the label after “EXP”. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Nebido contains
The active substance is testosterone undecanoate 250 mg/ml (corresponding to 157.9 mg testosterone). One ampoule / vial contains 1000 mg testosterone undecanoate (corresponding to 631.5 mg testosterone).
The other ingredients are benzyl benzoate and refined castor oil.
What Nebido looks like and contents of the pack
Nebido is a clear, yellowish oily liquid. The contents of the packs are:
1 brown glass ampoule / brown glass vial with 4 ml solution for injection
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Bayer plc
Bayer House
Strawberry Hill
Newbury
Berkshire RG14 1JA United Kingdom Manufacturer
Bayer Pharma AG
D - 13342 Berlin
Germany
This medicinal product is authorised in the Member
States of the EEA under the following names:
► Cyprus, Czech Republic, Greece, Denmark, Estonia, Latvia, Luxembourg, Malta, Poland and Portugal:
Nebido
► Austria: Nebido 1000 mg/4 ml Injektionslosung
► Belgium: Nebido 1000 mg/4 ml, oplossing voor injectie
► Croatia: Nebido 1000 mg/4 ml otopina za injekciju
► Finland: Nebido 1000 mg/4 ml injektioneste, liuos
► France: Nebido 1000 mg/4 ml, solution injectable
► Germany: Nebido 1000 mg Injektionslosung
► Hungary: Nebido 250 mg/ml oldatos injekcio
► Iceland: Nebido 1000 mg/4 ml stungulyf, lausn
► Italy: NEBID 1000 mg/4ml soluzione iniettabile
► Lithuania: Nebido 1000 mg/4 ml injekcinis tirpalas
► Netherlands: Nebido 1000 mg/4 ml
► Norway: Nebido 1000 mg/4 ml injeksjonsvxske, opplosning
► Slovak Republic: Nebido 1000 mg/4 ml injekcny roztok
► Slovenia: Nebido 1000 mg/4 ml raztopina za injiciranje
► Spain: REANDRON 1000 MG/ 4 ML SOLUCION INYECTABLE
► Sweden: Nebido, 1000 mg/4 ml injektionsvatska, losning
► UK and Ireland: Nebido 1000mg/4ml, solution for injection
This leaflet was last revised in January 2016
*
0
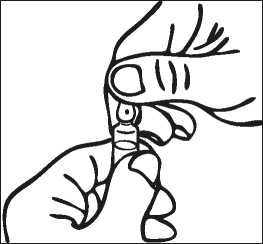
Notes on handling the OPC (One-Point-Cut) ampoule:
There is a pre-scored mark beneath the coloured point on the ampoule eliminating the need to file the neck. Prior to opening, ensure that any solution in the upper part of the ampoule flows down to the lower part. Use both hands to open; while holding the lower part of the ampoule in one hand, use the other hand to break off the upper part of the ampoule in the direction away from the coloured point.
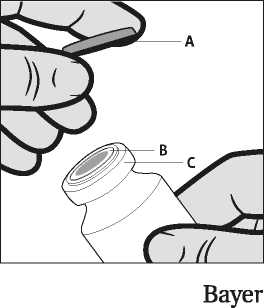
Notes on handling the vial
The vial is for single use only. The content of a vial is to be injected intramuscularly immediately after drawing up into the syringe. After removal of the plastic cap (A) do not remove the metal ring (B) or the crimp cap (C).
85065211_06.indd 2
05.04.2016 07:36:25